Alimera Sciences
Top View
- 02/10/2015 Provider Subsystem Healthcare and Family Services Run Time: 20:51:09 Report Id 2794D051 Page: 01
- Medicaid System (Mmis) Illinois Department of Run Date: 02/10/2015 Provider Subsystem Healthcare and Family Services Run Time: 20:51:09 Report Id 2794D052 Page: 01
- Alimera Sciences Inc
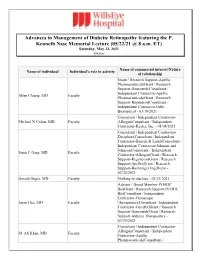
- Presenter Financial Disclosures for AAO 2020
- Retina Update: the 10Th Annual J
- Psivida Announces Phase I/II Clinical Study Evaluating Bioerodible, Sustained Release Latanoprost Device in Ocular Hypertension and Glaucoma
- Active Labelers Run Date : Aug 26, 2021
- A Comparison of the Efficacy of Brolucizumab and Aflibercept in Eyes with Early Persistent Retinal Fluid: 96–Week Results from the HAWK and HARRIER Studies
- Taking the Right Measures to Control COVID-19 in Ophthalmology: the Experience of a Tertiary Eye Care Referral Center in Italy
- THREE PILLARS of SUCCESS in Patient and Practice Management Strategies in Diabetic Macular Edema
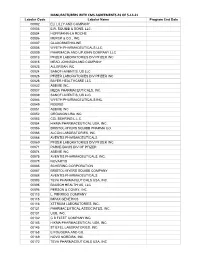
- 4Q2015 Active Labeler Labeler Company 00002 ELI LILLY and COMPANY 00003 E.R
- Customer Rebate List Covering
- 2020 Membership Benefits Guide
- Alimera Sciences. Page 2 of 20 [email protected]
- The Pharma 1000 Top Global Pharmaceutical Company Report
- List of Section 13F Securities, Fourth Quarter 2019
- Alimera Sciences Inc
- Draft Scope (Post-Referral)