A Comparison of the Efficacy of Brolucizumab and Aflibercept in Eyes with Early Persistent Retinal Fluid: 96–Week Results from the HAWK and HARRIER Studies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DRUGS REQUIRING PRIOR AUTHORIZATION in the MEDICAL BENEFIT Page 1
Effective Date: 08/01/2021 DRUGS REQUIRING PRIOR AUTHORIZATION IN THE MEDICAL BENEFIT Page 1 Therapeutic Category Drug Class Trade Name Generic Name HCPCS Procedure Code HCPCS Procedure Code Description Anti-infectives Antiretrovirals, HIV CABENUVA cabotegravir-rilpivirine C9077 Injection, cabotegravir and rilpivirine, 2mg/3mg Antithrombotic Agents von Willebrand Factor-Directed Antibody CABLIVI caplacizumab-yhdp C9047 Injection, caplacizumab-yhdp, 1 mg Cardiology Antilipemic EVKEEZA evinacumab-dgnb C9079 Injection, evinacumab-dgnb, 5 mg Cardiology Hemostatic Agent BERINERT c1 esterase J0597 Injection, C1 esterase inhibitor (human), Berinert, 10 units Cardiology Hemostatic Agent CINRYZE c1 esterase J0598 Injection, C1 esterase inhibitor (human), Cinryze, 10 units Cardiology Hemostatic Agent FIRAZYR icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent HAEGARDA c1 esterase J0599 Injection, C1 esterase inhibitor (human), (Haegarda), 10 units Cardiology Hemostatic Agent ICATIBANT (generic) icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent KALBITOR ecallantide J1290 Injection, ecallantide, 1 mg Cardiology Hemostatic Agent RUCONEST c1 esterase J0596 Injection, C1 esterase inhibitor (recombinant), Ruconest, 10 units Injection, lanadelumab-flyo, 1 mg (code may be used for Medicare when drug administered under Cardiology Hemostatic Agent TAKHZYRO lanadelumab-flyo J0593 direct supervision of a physician, not for use when drug is self-administered) Cardiology Pulmonary Arterial Hypertension EPOPROSTENOL (generic) -

BLA 761125 Page 7
BLA 761125 Page 7 HIGHLIGHTS OF PRESCRIBING INFORMATION -----------------------WARNINGS AND PRECAUTIONS---------------------- These highlights do not include all the information needed to use BEOVU Endophthalmitis and retinal detachments may occur following intravitreal safely and effectively. See full prescribing information for BEOVU. injections. Patients should be instructed to report any symptoms suggestive of endophthalmitis or retinal detachment without delay (5.1). BEOVU® (brolucizumab-dbll) injection, for intravitreal injection Increases in intraocular pressure (IOP) have been seen within 30 minutes of Initial U.S. Approval: 2019 an intravitreal injection (5.2). ----------------------------INDICATIONS AND USAGE------------------------- There is a potential risk of arterial thromboembolic events (ATE) following BEOVU is a human vascular endothelial growth factor (VEGF) inhibitor intravitreal use of VEGF inhibitors (5.3). indicated for the treatment of Neovascular (Wet) Age-Related Macular ------------------------------ADVERSE REACTIONS----------------------------- Degeneration (AMD) (1). The most common adverse reactions (≥ 5%) reported in patients receiving ----------------------DOSAGE AND ADMINISTRATION---------------------- BEOVU are vision blurred (10%), cataract (7%), conjunctival hemorrhage BEOVU is administered by intravitreal injection. The recommended dose for (6%), eye pain (5%), and vitreous floaters (5%) (6.1). BEOVU is 6 mg (0.05 mL of 120 mg/mL solution) monthly (approximately To report SUSPECTED ADVERSE REACTIONS, contact Novartis every 25-31 days) for the first three doses, followed by one dose of 6 mg (0.05 Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA mL) every 8-12 weeks (2). 1088 or www.fda.gov/medwatch. ---------------------DOSAGE FORMS AND STRENGTHS-------------------- See 17 for PATIENT COUNSELING INFORMATION. Injection: 6 mg/0.05 mL solution for intravitreal injection in a single-dose vial (3). -

NASDAQ Stock Market
Nasdaq Stock Market Friday, December 28, 2018 Name Symbol Close 1st Constitution Bancorp FCCY 19.75 1st Source SRCE 40.25 2U TWOU 48.31 21st Century Fox Cl A FOXA 47.97 21st Century Fox Cl B FOX 47.62 21Vianet Group ADR VNET 8.63 51job ADR JOBS 61.7 111 ADR YI 6.05 360 Finance ADR QFIN 15.74 1347 Property Insurance Holdings PIH 4.05 1-800-FLOWERS.COM Cl A FLWS 11.92 AAON AAON 34.85 Abiomed ABMD 318.17 Acacia Communications ACIA 37.69 Acacia Research - Acacia ACTG 3 Technologies Acadia Healthcare ACHC 25.56 ACADIA Pharmaceuticals ACAD 15.65 Acceleron Pharma XLRN 44.13 Access National ANCX 21.31 Accuray ARAY 3.45 AcelRx Pharmaceuticals ACRX 2.34 Aceto ACET 0.82 Achaogen AKAO 1.31 Achillion Pharmaceuticals ACHN 1.48 AC Immune ACIU 9.78 ACI Worldwide ACIW 27.25 Aclaris Therapeutics ACRS 7.31 ACM Research Cl A ACMR 10.47 Acorda Therapeutics ACOR 14.98 Activision Blizzard ATVI 46.8 Adamas Pharmaceuticals ADMS 8.45 Adaptimmune Therapeutics ADR ADAP 5.15 Addus HomeCare ADUS 67.27 ADDvantage Technologies Group AEY 1.43 Adobe ADBE 223.13 Adtran ADTN 10.82 Aduro Biotech ADRO 2.65 Advanced Emissions Solutions ADES 10.07 Advanced Energy Industries AEIS 42.71 Advanced Micro Devices AMD 17.82 Advaxis ADXS 0.19 Adverum Biotechnologies ADVM 3.2 Aegion AEGN 16.24 Aeglea BioTherapeutics AGLE 7.67 Aemetis AMTX 0.57 Aerie Pharmaceuticals AERI 35.52 AeroVironment AVAV 67.57 Aevi Genomic Medicine GNMX 0.67 Affimed AFMD 3.11 Agile Therapeutics AGRX 0.61 Agilysys AGYS 14.59 Agios Pharmaceuticals AGIO 45.3 AGNC Investment AGNC 17.73 AgroFresh Solutions AGFS 3.85 -
Advances in Management of Diabetic Retinopathy Featuring the P
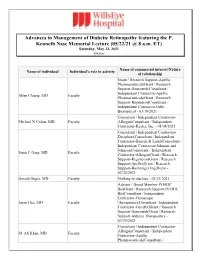
Advances in Management of Diabetic Retinopathy featuring the P. Kenneth Nase Memorial Lecture (05/22/21 @ 8 a.m. ET) Saturday, May 22, 2021 Online Name of commercial interest/Nature Name of individual Individual's role in activity of relationship Grant / Research Support-Apellis Pharmaceuticals|Grant / Research Support-Genentech|Consultant / Independent Contractor-Apellis Allen Chiang, MD Faculty Pharmaceuticals|Grant / Research Support-Regeneron|Consultant / Independent Contractor-Orbit Biomedical - 03/19/2021 Consultant / Independent Contractor- Michael N Cohen, MD Faculty Allergan|Consultant / Independent Contractor-Keeler, Inc. - 05/04/2021 Consultant / Independent Contractor- Deciphera|Consultant / Independent Contractor-Bausch & Lomb|Consultant / Independent Contractor-Johnson and Johnson|Consultant / Independent Sunir J. Garg, MD Faculty Contractor-Allergan|Grant / Research Support-Regeneron|Grant / Research Support-Apellis|Grant / Research Support-Boehringer Ingelheim - 02/24/2021 Omesh Gupta, MD Faculty Nothing to disclose - 03/21/2021 Advisor / Board Member-IVERIC Bio|Grant / Research Support-IVERIC Bio|Consultant / Independent Contractor-Gyroscope Jason Hsu, MD Faculty Therapeutics|Consultant / Independent Contractor-OccuRx|Grant / Research Support-Genentech|Grant / Research Support-Aldeyra Therapeutics - 03/20/2021 Consultant / Independent Contractor- Allergan|Consultant / Independent M. Ali Khan, MD Faculty Contractor-Apellis Pharmaceuticals|Consultant / Independent Contractor- Genentech|Grant / Research Support- Regeneron -

Medicaid System (Mmis) Illinois Department of Run Date: 08/08/2015 Provider Subsystem Healthcare and Family Services Run Time: 21:25:58 Report Id 2794D052 Page: 01
MEDICAID SYSTEM (MMIS) ILLINOIS DEPARTMENT OF RUN DATE: 08/08/2015 PROVIDER SUBSYSTEM HEALTHCARE AND FAMILY SERVICES RUN TIME: 21:25:58 REPORT ID 2794D052 PAGE: 01 ALPHA COMPLETE LIST OF PHARMACEUTICAL LABELERS WITH SIGNED REBATE AGREEMENTS IN EFFECT AS OF 10/01/2015 NDC NDC PREFIX LABELER NAME PREFIX LABELER NAME 68782 (OSI) EYETECH 65162 AMNEAL PHARMACEUTICALS LLC 00074 ABBOTT LABORATORIES 69238 AMNEAL PHARMACEUTICALS, LLC 68817 ABRAXIS BIOSCIENCE, LLC 53150 AMNEAL-AGILA, LLC 16729 ACCORD HEALTHCARE INCORPORATED 00548 AMPHASTAR PHARMACEUTICALS, INC. 42192 ACELLA PHARMACEUTICALS, LLC 66780 AMYLIN PHARMACEUTICALS, INC. 10144 ACORDA THERAPEUTICS, INC. 55724 ANACOR PHARMACEUTICALS 00472 ACTAVIS 10370 ANCHEN PHARMACEUTICALS, INC. 00228 ACTAVIS ELIZABETH LLC 62559 ANIP ACQUISITION COMPANY 45963 ACTAVIS INC. 54436 ANTARES PHARMA, INC. 46987 ACTAVIS KADIAN LLC 52609 APO-PHARMA USA, INC. 49687 ACTAVIS KADIAN LLC 60505 APOTEX CORP. 14550 ACTAVIS PHARMA MFGING PRIVATE LIMITED 63323 APP PHARMACEUTICALS, LLC. 67767 ACTAVIS SOUTH ATLANTIC 42865 APTALIS PHARMA US, INC 66215 ACTELION PHARMACEUTICALS U.S., INC. 58914 APTALIS PHARMA US, INC. 52244 ACTIENT PHARMACEUTICALS 13310 AR SCIENTIFIC, INC. 75989 ACTON PHARMACEUTICALS 08221 ARBOR PHARM IRELAND LIMITED 76431 AEGERION PHARMACEUTICALS, INC. 60631 ARBOR PHARMACEUTICALS IRELAND LIMITED 50102 AFAXYS, INC. 24338 ARBOR PHARMACEUTICALS, INC. 10572 AFFORDABLE PHARMACEUTICALS, LLC 59923 AREVA PHARMACEUTICALS 27241 AJANTA PHARMA LIMITED 76189 ARIAD PHARMACEUTICALS, INC. 17478 AKORN INC 24486 ARISTOS PHARMACEUTICALS, INC. 24090 AKRIMAX PHARMACEUTICALS LLC 67877 ASCEND LABORATORIES, L.L.C. 68220 ALAVEN PHARMACEUTICAL, LLC 76388 ASPEN GLOBAL INC. 00065 ALCON LABORATORIES, INC. 51248 ASTELLAS 00998 ALCON LABORATORIES, INC. 00469 ASTELLAS PHARMA US, INC. 25682 ALEXION PHARMACEUTICALS 00186 ASTRAZENECA LP 68611 ALIMERA SCIENCES, INC. -

Regeneron Needs a New Plan B for Eylea
November 27, 2017 Regeneron needs a new plan B for Eylea Madeleine Armstrong Regeneron’s efforts to extend the lifespan of its blockbuster Eylea franchise via combinations have fallen flat again, raising the question of whether it can find anything that can best Eylea alone. With competition on the horizon from Novartis’s rival wet age-related macular degeneration (AMD) project, brolucizumab, Eylea sales are forecast to flatten after 2020 – something that Regeneron cannot now counter with combos (see table below). Regeneron’s stock was down 3% in premarket trading this morning, but the shares opened down just 1%. Investors might have been reassured by the fact that full data from the Hawk and Harrier studies of brolucizumab, released earlier this month, were not the smash hit that Novartis had hoped for. This could allow Eylea to keep its dominant position in the wet age-related macular degeneration (AMD) market for some time to come. Top wet AMD drugs in 2022 Annual indication sales ($m) Product Company Status 2016 2018e 2020e 2022e Eylea Regeneron/Bayer/Santen Pharmaceutical Marketed 3,584 3,875 4,073 3,942 Lucentis Novartis/Roche Marketed 2,354 2,271 1,924 1,460 Brolucizumab Novartis Phase III - - 319 937 Source: EvaluatePharma. And if positive, the phase III Panorama study of Eylea monotherapy in diabetic retinopathy, due to report in the first half of next year, could open up another avenue for growth for the company’s most valuable product. But even the most optimistic Regeneron bulls will have to admit that Eylea’s sales are likely to be eroded by the market entry of brolucizumab, expected in 2019 or 2020. -

The Two Tontti Tudiul Lui Hi Ha Unit
THETWO TONTTI USTUDIUL 20170267753A1 LUI HI HA UNIT ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2017 /0267753 A1 Ehrenpreis (43 ) Pub . Date : Sep . 21 , 2017 ( 54 ) COMBINATION THERAPY FOR (52 ) U .S . CI. CO - ADMINISTRATION OF MONOCLONAL CPC .. .. CO7K 16 / 241 ( 2013 .01 ) ; A61K 39 / 3955 ANTIBODIES ( 2013 .01 ) ; A61K 31 /4706 ( 2013 .01 ) ; A61K 31 / 165 ( 2013 .01 ) ; CO7K 2317 /21 (2013 . 01 ) ; (71 ) Applicant: Eli D Ehrenpreis , Skokie , IL (US ) CO7K 2317/ 24 ( 2013. 01 ) ; A61K 2039/ 505 ( 2013 .01 ) (72 ) Inventor : Eli D Ehrenpreis, Skokie , IL (US ) (57 ) ABSTRACT Disclosed are methods for enhancing the efficacy of mono (21 ) Appl. No. : 15 /605 ,212 clonal antibody therapy , which entails co - administering a therapeutic monoclonal antibody , or a functional fragment (22 ) Filed : May 25 , 2017 thereof, and an effective amount of colchicine or hydroxy chloroquine , or a combination thereof, to a patient in need Related U . S . Application Data thereof . Also disclosed are methods of prolonging or increasing the time a monoclonal antibody remains in the (63 ) Continuation - in - part of application No . 14 / 947 , 193 , circulation of a patient, which entails co - administering a filed on Nov. 20 , 2015 . therapeutic monoclonal antibody , or a functional fragment ( 60 ) Provisional application No . 62/ 082, 682 , filed on Nov . of the monoclonal antibody , and an effective amount of 21 , 2014 . colchicine or hydroxychloroquine , or a combination thereof, to a patient in need thereof, wherein the time themonoclonal antibody remains in the circulation ( e . g . , blood serum ) of the Publication Classification patient is increased relative to the same regimen of admin (51 ) Int . -

(12) Patent Application Publication (10) Pub. No.: US 2017/0172932 A1 Peyman (43) Pub
US 20170172932A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0172932 A1 Peyman (43) Pub. Date: Jun. 22, 2017 (54) EARLY CANCER DETECTION AND A 6LX 39/395 (2006.01) ENHANCED IMMUNOTHERAPY A61R 4I/00 (2006.01) (52) U.S. Cl. (71) Applicant: Gholam A. Peyman, Sun City, AZ CPC .......... A61K 9/50 (2013.01); A61K 39/39558 (US) (2013.01); A61K 4I/0052 (2013.01); A61 K 48/00 (2013.01); A61K 35/17 (2013.01); A61 K (72) Inventor: sham A. Peyman, Sun City, AZ 35/15 (2013.01); A61K 2035/124 (2013.01) (21) Appl. No.: 15/143,981 (57) ABSTRACT (22) Filed: May 2, 2016 A method of therapy for a tumor or other pathology by administering a combination of thermotherapy and immu Related U.S. Application Data notherapy optionally combined with gene delivery. The combination therapy beneficially treats the tumor and pre (63) Continuation-in-part of application No. 14/976,321, vents tumor recurrence, either locally or at a different site, by filed on Dec. 21, 2015. boosting the patient’s immune response both at the time or original therapy and/or for later therapy. With respect to Publication Classification gene delivery, the inventive method may be used in cancer (51) Int. Cl. therapy, but is not limited to such use; it will be appreciated A 6LX 9/50 (2006.01) that the inventive method may be used for gene delivery in A6 IK 35/5 (2006.01) general. The controlled and precise application of thermal A6 IK 4.8/00 (2006.01) energy enhances gene transfer to any cell, whether the cell A 6LX 35/7 (2006.01) is a neoplastic cell, a pre-neoplastic cell, or a normal cell. -

International Nonproprietary Names for Pharmaceutical Substances (INN)
pre-publication copy Recommended INN: List 74 International Nonproprietary Names for Pharmaceutical Substances (INN) RECOMMENDED International Nonproprietary Names: List 74 Notice is hereby given that, in accordance with paragraph 7 of the Procedure for the Selection of Recommended International Nonproprietary Names for Pharmaceutical Substances [Off. Rec. Wld Health Org., 1955, 60, 3 (Resolution EB15.R7); 1969, 173, 10 (Resolution EB43.R9); Resolution EB115.R4 (EB115/2005/REC/1)], the following names are selected as Recommended International Nonproprietary Names. The inclusion of a name in the lists of Recommended International Nonproprietary Names does not imply any recommendation of the use of the substance in medicine or pharmacy. Lists of Proposed (1–109) and Recommended (1–70) International Nonproprietary Names can be found in Cumulative List No. 15, 2013 (available in CD-ROM only). Dénominations communes internationales des Substances pharmaceutiques (DCI) Dénominations communes internationales RECOMMANDÉES: Liste 74 Il est notifié que, conformément aux dispositions du paragraphe 7 de la Procédure à suivre en vue du choix de Dénominations communes internationales recommandées pour les Substances pharmaceutiques [Actes off. Org. mond. Santé, 1955, 60, 3 (résolution EB15.R7); 1969, 173, 10 (résolution EB43.R9); résolution EB115.R4 (EB115/2005/REC/1)] les dénominations ci-dessous sont choisies par l’Organisation mondiale de la Santé en tant que dénominations communes internationales recommandées. L’inclusion d’une dénomination dans les listes de DCI recommandées n’implique aucune recommandation en vue de l’utilisation de la substance correspondante en médecine ou en pharmacie. On trouvera d’autres listes de Dénominations communes internationales proposées (1–109) et recommandées (1–70) dans la Liste récapitulative No. -

2019 FDA Biopharmaceutical Approvals: a Record Year for Follow-On Products, but Is Innovation Lagging? Bioplan Associates, Inc
Confidential White Paper – Not for Distribution 2019 FDA Biopharmaceutical Approvals: A Record Year for Follow-on Products, But is Innovation Lagging? BioPlan Associates, Inc. January 2020 Introduction Although 2019 was overall a good year for biopharmaceutical product approvals, there was a lower percentage of approvals for fully new, innovative products compared with prior years. This may indicate problems with recent years’ pharmaceutical industry increased investment in biopharmaceutical vs. drug R&D, with a relative increase in resulting innovative product approvals not showing up yet. It may be too early to make conclusions regarding the extent of this pipeline trend, but the record portion of follow-on product approvals (63% of approvals) can be viewed as positive or negative for the industry. Biopharmaceuticals are here defined as prescription therapeutic products containing active agents manufactured using biotechnology methods, i.e., using living organisms (1). Figure 1 shows the numbers of recombinant and non-recombinant biopharmaceuticals approved by FDA since 1981, when the first recombinant protein received approval. Total biopharmaceutical approvals have increased over the years while remaining steady in the past few years. Figure 1: Numbers of Recombinant and Non-Recombinant Biopharmaceuticals Approved by FDA, 1981-2019 ©2020 BioPlan Associates, Inc, 2275 Research Blvd., Ste. 500, Rockville, MD 20850 (301) 921-5979 Page: 1 Confidential White Paper – Not for Distribution Approvals in 2019 In Table 1 we present approvals during 2019. Several oligonucleotide therapeutics, both antisense and RNAi, also received approval, but these are synthetically manufactured drugs and not considered biopharmaceuticals. Other products not included as biopharmaceuticals include medical devices, allergenic extract products, and diagnostics that receive biologics (BLA) approvals. -

CHMP Agenda of the 19-22 April 2021 Meeting
28 July 2021 EMA/CHMP/220334/2021 Corr.11 Human Medicines Division Committee for medicinal products for human use (CHMP) Agenda for the meeting on 19-22 April 2021 Chair: Harald Enzmann – Vice-Chair: Bruno Sepodes 19 April 2021, 09:00 – 19:30, virtual meeting/ room 1C 20 April 2021, 08:30 – 19:30, virtual meeting/ room 1C 21 April 2021, 08:30 – 19:30, virtual meeting/ room 1D 22 April 2021, 08:30 – 19:00, virtual meeting/ room 1C Disclaimers Some of the information contained in this agenda is considered commercially confidential or sensitive and therefore not disclosed. With regard to intended therapeutic indications or procedure scopes listed against products, it must be noted that these may not reflect the full wording proposed by applicants and may also vary during the course of the review. Additional details on some of these procedures will be published in the CHMP meeting highlights once the procedures are finalised and start of referrals will also be available. Of note, this agenda is a working document primarily designed for CHMP members and the work the Committee undertakes. Note on access to documents Some documents mentioned in the agenda cannot be released at present following a request for access to documents within the framework of Regulation (EC) No 1049/2001 as they are subject to on- going procedures for which a final decision has not yet been adopted. They will become public when adopted or considered public according to the principles stated in the Agency policy on access to documents (EMA/127362/2006). 1 Correction in section 8.1.1 Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2021. -

Heparin-Binding VEGFR1 Variants As Long-Acting VEGF Inhibitors for Treatment of Intraocular Neovascular Disorders
Heparin-binding VEGFR1 variants as long-acting VEGF inhibitors for treatment of intraocular neovascular disorders Hong Xina, Nilima Biswasa, Pin Lia, Cuiling Zhonga, Tamara C. Chanb, Eric Nudlemanb, and Napoleone Ferraraa,b,1 aDepartment of Pathology, University of California San Diego, La Jolla, CA 92093; and bDepartment of Ophthalmology, University of California San Diego, La Jolla, CA 92093 Contributed by Napoleone Ferrara, April 20, 2021 (sent for review December 4, 2019; reviewed by Jayakrishna Ambati and Lois E. H. Smith) Neovascularization is a key feature of ischemic retinal diseases and VEGF inhibitors have become a standard of therapy in mul- the wet form of age-related macular degeneration (AMD), all lead- tiple tumors and have transformed the treatment of intraocular ing causes of severe vision loss. Vascular endothelial growth factor neovascular disorders such as the neovascular form of age-related (VEGF) inhibitors have transformed the treatment of these disor- macular degeneration (AMD), proliferative diabetic retinopathy, ders. Millions of patients have been treated with these drugs and retinal vein occlusion, which are leading causes of severe vision worldwide. However, in real-life clinical settings, many patients loss and legal blindness (3, 5, 18). Currently, three anti-VEGF drugs do not experience the same degree of benefit observed in clinical are widely used in the United States for ophthalmological indica- trials, in part because they receive fewer anti-VEGF injections. tions: bevacizumab, ranibizumab, and aflibercept (3). Bevacizumab Therefore, there is an urgent need to discover and identify novel is a full-length IgG antibody targeting VEGF (19). Even though long-acting VEGF inhibitors.