The Pharma 1000 Top Global Pharmaceutical Company Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Kopi Af Aktivlisten 2021-06-30 Ny.Xlsm
Velliv noterede aktier i alt pr. 30-06-2021 ISIN Udstedelsesland Navn Markedsværdi (i DKK) US0378331005 US APPLE INC 1.677.392.695 US5949181045 US MICROSOFT CORP 1.463.792.732 US0231351067 US AMAZON.COM INC 1.383.643.996 DK0060534915 DK NOVO NORDISK A/S-B 1.195.448.146 US30303M1027 US FACEBOOK INC-CLASS A 1.169.094.867 US02079K3059 US ALPHABET INC-CL A 867.740.769 DK0010274414 DK DANSKE BANK A/S 761.684.457 DK0060079531 DK DSV PANALPINA A/S 629.313.827 US02079K1079 US ALPHABET INC-CL C 589.305.120 US90138F1021 US TWILIO INC - A 514.807.852 US57636Q1040 US MASTERCARD INC - A 490.766.560 US4781601046 US JOHNSON & JOHNSON 478.682.981 US70450Y1038 US PAYPAL HOLDINGS INC 471.592.728 DK0061539921 DK VESTAS WIND SYSTEMS A/S 441.187.698 US79466L3024 US SALESFORCE.COM INC 439.114.061 US01609W1027 US ALIBABA GROUP HOLDING-SP ADR 432.325.255 US8835561023 US THERMO FISHER SCIENTIFIC INC 430.036.612 US22788C1053 US CROWDSTRIKE HOLDINGS INC - A 400.408.622 KYG875721634 HK TENCENT HOLDINGS LTD 397.054.685 KR7005930003 KR SAMSUNG ELECTRONICS CO LTD 389.413.700 DK0060094928 DK ORSTED A/S 378.578.374 ES0109067019 ES AMADEUS IT GROUP SA 375.824.429 US46625H1005 US JPMORGAN CHASE & CO 375.282.618 US67066G1040 US NVIDIA CORP 357.034.119 US17275R1023 US CISCO SYSTEMS INC 348.160.692 DK0010244508 DK AP MOLLER-MAERSK A/S-B 339.783.859 US20030N1019 US COMCAST CORP-CLASS A 337.806.502 NL0010273215 NL ASML HOLDING NV 334.040.559 CH0012032048 CH ROCHE HOLDING AG-GENUSSCHEIN 325.008.200 KYG970081173 HK WUXI BIOLOGICS CAYMAN INC 321.300.236 US4370761029 US HOME DEPOT INC 317.083.124 US58933Y1055 US MERCK & CO. -

Serbia – Montenegro
GEF Pipeline Entry Project Concept and PDF-B Request Country: Serbia and Montenegro Project Title: Serbia: Reduction of Enterprise Nutrient Discharges Project (RENDR) (under the WB-GEF Investment Fund for Nutrient Reduction in the Black Sea/Danube Basin) GEF Implementing Agency: World Bank GEF Focal Area: International Waters GEF Operational Program: OP. 8 Waterbody-based Operational Program GEF Grant Amount: USD 6-9 million Total Project Cost: USD 12-18 million PDF-B Requested: US$ 350,000 PDF-B Cofinancing: US$ 35,000 (from government) Executing Agency: Ministry of Environment and Natural Resources of Serbia Implementation Start: January 2005 Duration: 5 years 1. Project Summary Serbia is among the largest nutrient polluters of the Danube River and enterprises, notably agro-processing and large scale livestock breeding farms are major sources of pollution. The global environment objective of the Reduction of Enterprise Nutrient Discharges Project would be to reduce nutrient pollution from hotspot enterprises located in the Republic of Serbia. This would also help the country of Serbia and Montenegro (SAM, the union of the Republic of Serbia and the Republic of Montenegro) meet its international commitments under the Danube River Convention. The development objective would be to reduce the negative public health, economic and amenity impact associated with water and soil pollution from enterprise pollutant discharges. The proposed project would consist of four components: (i) Regulatory Reform and Capacity Building; (ii) Investment in Industrial Nutrient Reduction (incl. fertilizer factories, agro-processors, and large-scale livestock farms); (iii) Awareness Raising and Replicability Strategy; and (iv) Project Management and Monitoring. 2. Country Ownership (a) Country Eligibility SAM has signed (2002) and ratified (2003) the Convention on Cooperation for the Protection and Sustainable Use of the Danube River (Danube Convention) (1994). -

The Anthony F. Farma, CFP® Scholarship Application FPA of Massachusetts
The Anthony F. Farma, CFP® Scholarship Application FPA of Massachusetts The primary aim of the Financial Planning Association™ (FPA™) is to be a community that fosters the value of financial planning and advances the financial planning profession. FPA’s strategy to accomplish its objectives involves welcoming all those who advance the financial planning process ® and promoting the CFP mark as the cornerstone of the financial planning profession. Award Recipient of the Anthony F. Farma, CFP® Scholarship will receive: ® § a $500 tuition scholarship (payable to CFP education program/school) § FPA National Membership Enrollment for 1 year § FPAMA Local Membership Enrollment for 1 year § FPA MA 3 meeting season pass (includes February, October, and December meetings) plus the May Annual Conference. Application Instructions for Merit Based Scholarship Eligibility To be eligible an applicant must meet all of the following qualifications: ® § Currently enrolled in a CFP program administered by an accredited university or college registered with the CFP® Board of Standards. (The scholarship may be used towards a review course.) ® § Have the intention to become a CFP certificant by fulfilling all requirements (currently six courses) and sitting for the CFP® Board of Standards comprehensive certification examination. § Must demonstrate academic and/or professional accomplishment, e.g., GPA, degrees received, and/or specific professional achievements. ® § Furnish one letter of recommendation. (Consider professors, instructors, CFP practitioners, -

EP Vantage Interview - Silence Hoping to Have Something to Shout About in 2009
December 22, 2008 EP Vantage Interview - Silence hoping to have something to shout about in 2009 Lisa Urquhart With its first product expected to go into the clinic potentially as early as January, Silence Therapeutics is hoping that 2009 will be year it has something to shout about and one that will reverse the alarming share price decline that has seen the company’s valuation slip from a high of over £170m in June 2007 to £21.6m today. Silence is one of a growing number of companies working in RNA interference (RNAi) and particularly short interfering RNA (siRNA), which work by selectively silencing or inactivating genes related to certain diseases. It is importantly one of only two companies that have composition of matter patent protection for their siRNA drugs. Speaking to EP Vantage, Iain Ross, chief executive of Silence, says: “It’s a big year coming up for us.” The group has spent most of the last 12 months strengthening its IP position, and next year comes the important move of the group’s lead candidate Atu027 into the clinic, an event Mr Ross says should take place in the first quarter of the year. Many expect it could be as soon as the end of January. Partnering focus What is less expected is the speed at which Mr Ross intends to partner the drug, which is being developed in solid tumours, with an emphasis on lung cancer. “We would be looking at doing something either at the end of 2009 or the beginning of 2010,” he says. Key to partnering discussions will be the drug demonstrating good safety data in a number of cancer patients, which could be the catalyst to starting talks with big pharma who have already started to ask about Atu027. -

NASDAQ Stock Market
Nasdaq Stock Market Friday, December 28, 2018 Name Symbol Close 1st Constitution Bancorp FCCY 19.75 1st Source SRCE 40.25 2U TWOU 48.31 21st Century Fox Cl A FOXA 47.97 21st Century Fox Cl B FOX 47.62 21Vianet Group ADR VNET 8.63 51job ADR JOBS 61.7 111 ADR YI 6.05 360 Finance ADR QFIN 15.74 1347 Property Insurance Holdings PIH 4.05 1-800-FLOWERS.COM Cl A FLWS 11.92 AAON AAON 34.85 Abiomed ABMD 318.17 Acacia Communications ACIA 37.69 Acacia Research - Acacia ACTG 3 Technologies Acadia Healthcare ACHC 25.56 ACADIA Pharmaceuticals ACAD 15.65 Acceleron Pharma XLRN 44.13 Access National ANCX 21.31 Accuray ARAY 3.45 AcelRx Pharmaceuticals ACRX 2.34 Aceto ACET 0.82 Achaogen AKAO 1.31 Achillion Pharmaceuticals ACHN 1.48 AC Immune ACIU 9.78 ACI Worldwide ACIW 27.25 Aclaris Therapeutics ACRS 7.31 ACM Research Cl A ACMR 10.47 Acorda Therapeutics ACOR 14.98 Activision Blizzard ATVI 46.8 Adamas Pharmaceuticals ADMS 8.45 Adaptimmune Therapeutics ADR ADAP 5.15 Addus HomeCare ADUS 67.27 ADDvantage Technologies Group AEY 1.43 Adobe ADBE 223.13 Adtran ADTN 10.82 Aduro Biotech ADRO 2.65 Advanced Emissions Solutions ADES 10.07 Advanced Energy Industries AEIS 42.71 Advanced Micro Devices AMD 17.82 Advaxis ADXS 0.19 Adverum Biotechnologies ADVM 3.2 Aegion AEGN 16.24 Aeglea BioTherapeutics AGLE 7.67 Aemetis AMTX 0.57 Aerie Pharmaceuticals AERI 35.52 AeroVironment AVAV 67.57 Aevi Genomic Medicine GNMX 0.67 Affimed AFMD 3.11 Agile Therapeutics AGRX 0.61 Agilysys AGYS 14.59 Agios Pharmaceuticals AGIO 45.3 AGNC Investment AGNC 17.73 AgroFresh Solutions AGFS 3.85 -

Downloads but Many Other Countries — Such As the RCMP Said the Policy Revisions /JJ Release on April30.Pdf)
CMAJ News For the record Published at www.cmaj.ca between Apr. The new warning letter regards in persistent or significant disability or 20 and May 18 breaches uncovered during a July– incapacity, be life-threatening or result August 2009 FDA inspection of an in death. Apotex receives second FDA Apotex facility located at 150 Signet The report indicates that there has Drive in Toronto, during which “sev- been a steady increase of adverse reac- warning eral violations that are identical” to tion reports since 2001, when roughly those found during the inspection of the 11 000 incidents were reported (www.hc he United States Food and Drug Etobicoke facility were discovered. -sc.gc.ca/dhp-mps/alt_formats/pdf/med Administration (FDA) has “These identical CGMP violations eff/bulletin/carn-bcei_v20n2-eng.pdf). T issued a second warning letter demonstrated a lack of adequate Roughly 70% (18 301) of 2009 inci- in a year to Toronto, Ontario-based process controls and raised serious dents involved pharamaceuticals, while generic drug manufacturer Apotex Inc. questions regarding your corporation’s 23% (5998) involved biotechnology for lapses in good manufacturing prac- quality and production systems,” the products. There were 833 adverse reac- tices. warning letter states. tion reports involving biologics, 516 The violations cause Apotex drug The violations included contamina- involving natural health products, 379 products to be considered “adulterated” tion of a diabetes drug with an “active involving radiopharmaceuticals and 34 within US regulations -
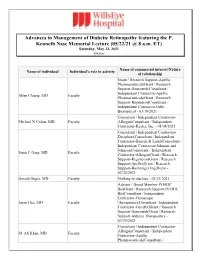
Advances in Management of Diabetic Retinopathy Featuring the P
Advances in Management of Diabetic Retinopathy featuring the P. Kenneth Nase Memorial Lecture (05/22/21 @ 8 a.m. ET) Saturday, May 22, 2021 Online Name of commercial interest/Nature Name of individual Individual's role in activity of relationship Grant / Research Support-Apellis Pharmaceuticals|Grant / Research Support-Genentech|Consultant / Independent Contractor-Apellis Allen Chiang, MD Faculty Pharmaceuticals|Grant / Research Support-Regeneron|Consultant / Independent Contractor-Orbit Biomedical - 03/19/2021 Consultant / Independent Contractor- Michael N Cohen, MD Faculty Allergan|Consultant / Independent Contractor-Keeler, Inc. - 05/04/2021 Consultant / Independent Contractor- Deciphera|Consultant / Independent Contractor-Bausch & Lomb|Consultant / Independent Contractor-Johnson and Johnson|Consultant / Independent Sunir J. Garg, MD Faculty Contractor-Allergan|Grant / Research Support-Regeneron|Grant / Research Support-Apellis|Grant / Research Support-Boehringer Ingelheim - 02/24/2021 Omesh Gupta, MD Faculty Nothing to disclose - 03/21/2021 Advisor / Board Member-IVERIC Bio|Grant / Research Support-IVERIC Bio|Consultant / Independent Contractor-Gyroscope Jason Hsu, MD Faculty Therapeutics|Consultant / Independent Contractor-OccuRx|Grant / Research Support-Genentech|Grant / Research Support-Aldeyra Therapeutics - 03/20/2021 Consultant / Independent Contractor- Allergan|Consultant / Independent M. Ali Khan, MD Faculty Contractor-Apellis Pharmaceuticals|Consultant / Independent Contractor- Genentech|Grant / Research Support- Regeneron -

ENDO INTERNATIONAL PLC (Exact Name of Registrant As Specified in Its Charter)
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Date of Report (Date of the earliest event reported): January 16, 2015 ENDO INTERNATIONAL PLC (Exact name of registrant as specified in its charter) Ireland 001-36326 Not Applicable (State or other jurisdiction of (Commission (I.R.S Employer incorporation or organization) File Number) Identification No.) Minerva House, Simmonscourt Road, Ballsbridge, Dublin 4, Ireland Not Applicable (Address of principal executive offices) (Zip Code) Registrant’s telephone number, including area code 011-353-1-268-2000 Not Applicable Former name or former address, if changed since last report Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions: x Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) ¨ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) ¨ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) ¨ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) Item 8.01 Other Events. This Current Report on Form 8-K is being filed pursuant to a memorandum of understanding regarding the settlement of certain litigation relating to the proposed merger (the “Merger”) between Auxilium Pharmaceuticals, Inc. (“Auxilium”) and Avalon Merger Sub Inc. (“Merger Sub”) pursuant to that certain Amended and Restated Agreement and Plan of Merger, dated as of November 17, 2014 (the “Merger Agreement”), by and among Auxilium, Endo International plc (“Endo”), Endo U.S. -

Md. Jury Awards $18M to Former Business Partner in Pharma Lawsuit – Maryland Daily Record
11/26/2018 Md. jury awards $18M to former business partner in pharma lawsuit – Maryland Daily Record Md. jury awards $18M to former business partner in pharma lawsuit By: Anamika Roy Daily Record Legal Affairs Writer November 26, 2018 A jury in a federal lawsuit awarded a Rockville scientist $18 million in a lawsuit against his former business partners. In a lawsuit filed in U.S. District Court in Greenbelt, Claudio De Simone and ExeGi Pharma LLC alleged VSL Pharmaceuticals Inc., Leadiant Biosciences Inc. and Alfasigma USA Inc. tried to make a copy of a drug De Simone created without having access to the proprietary formula and sold a “knock-off” version of the drug. De Simone also alleged the copy product had never been clinically tested, unlike his product, which underwent extensive clinical research, the lawsuit states. After a three-week trial and about 12 hours of deliberations, a jury of seven women and two men found last week that the pharmaceutical companies owe De Simone more than $18 million in damages, including $15 million for false advertising, nearly $1.9 million for unjust enrichment and nearly $1 million for breach of contract, according to online court records. An attorney for De Simone said Monday that the jury was likely swayed in favor of his client because of “the degree of willful misconduct” on the other side. “We proved that they were marketing to the general public, that includes some very sick patients who rely on the product my client invented to manage severe symptoms of gastrointestinal diseases,” said Jeremy W. -

US Pharma's Business Model
INNOVATION-FUELLED, SUSTAINABLE, INCLUSIVE GROWTH Working Paper US Pharma’s Business Model: Why It Is Broken, and How It Can Be Fixed William Lazonick Matt Hopkins Ken Jacobson Mustafa Erdem Sakinç Öner Tulum The Academic-Industry Research Network 13/2017 May This project has received funding from the European Union Horizon 2020 Research and Innovation action under grant agreement No 649186 US Pharma’s Business Model: Why It Is Broken, and How It Can Be Fixed William Lazonick Matt Hopkins Ken Jacobson Mustafa Erdem Sakinç Öner Tulum The Academic-Industry Research Network (www.theAIRnet.org) Revised, May 22, 2017 Chapter for inclusion in David Tyfield, Rebecca Lave, Samuel Randalls, and Charles Thorpe, eds., The Routledge Handbook of the Political Economy of Science The contents of this chapter are drawn from two contributions by the Academic- Industry Research Network to the United Nations Secretary-General’s High-Level Panel on Access to Medicines: http://www.unsgaccessmeds.org/list-of-contribution/ William Lazonick is Professor of Economics, University of Massachusetts Lowell; Visiting Professor, University of Ljubljana; Professeur Associé, Institut Mines- Télécom; and President, The Academic-Industry Research Network (theAIRnet); Matt Hopkins, Ken Jacobson, Mustafa Erdem Sakinç, and Öner Tulum are researchers at theAIRnet. Jacobson is also theAIRnet communications director. Sakinç has just completed a PhD in economics at the University of Bordeaux. Tulum is a PhD student at the University of Ljubljana. Funding for this research came from the Institute for New Economic Thinking (Collective and Cumulative Careers project), the European Union Horizon 2020 Research and Innovation Programme under grant agreement No. -

Global Equity Fund Description Plan 3S DCP & JRA MICROSOFT CORP
Global Equity Fund June 30, 2020 Note: Numbers may not always add up due to rounding. % Invested For Each Plan Description Plan 3s DCP & JRA MICROSOFT CORP 2.5289% 2.5289% APPLE INC 2.4756% 2.4756% AMAZON COM INC 1.9411% 1.9411% FACEBOOK CLASS A INC 0.9048% 0.9048% ALPHABET INC CLASS A 0.7033% 0.7033% ALPHABET INC CLASS C 0.6978% 0.6978% ALIBABA GROUP HOLDING ADR REPRESEN 0.6724% 0.6724% JOHNSON & JOHNSON 0.6151% 0.6151% TENCENT HOLDINGS LTD 0.6124% 0.6124% BERKSHIRE HATHAWAY INC CLASS B 0.5765% 0.5765% NESTLE SA 0.5428% 0.5428% VISA INC CLASS A 0.5408% 0.5408% PROCTER & GAMBLE 0.4838% 0.4838% JPMORGAN CHASE & CO 0.4730% 0.4730% UNITEDHEALTH GROUP INC 0.4619% 0.4619% ISHARES RUSSELL 3000 ETF 0.4525% 0.4525% HOME DEPOT INC 0.4463% 0.4463% TAIWAN SEMICONDUCTOR MANUFACTURING 0.4337% 0.4337% MASTERCARD INC CLASS A 0.4325% 0.4325% INTEL CORPORATION CORP 0.4207% 0.4207% SHORT-TERM INVESTMENT FUND 0.4158% 0.4158% ROCHE HOLDING PAR AG 0.4017% 0.4017% VERIZON COMMUNICATIONS INC 0.3792% 0.3792% NVIDIA CORP 0.3721% 0.3721% AT&T INC 0.3583% 0.3583% SAMSUNG ELECTRONICS LTD 0.3483% 0.3483% ADOBE INC 0.3473% 0.3473% PAYPAL HOLDINGS INC 0.3395% 0.3395% WALT DISNEY 0.3342% 0.3342% CISCO SYSTEMS INC 0.3283% 0.3283% MERCK & CO INC 0.3242% 0.3242% NETFLIX INC 0.3213% 0.3213% EXXON MOBIL CORP 0.3138% 0.3138% NOVARTIS AG 0.3084% 0.3084% BANK OF AMERICA CORP 0.3046% 0.3046% PEPSICO INC 0.3036% 0.3036% PFIZER INC 0.3020% 0.3020% COMCAST CORP CLASS A 0.2929% 0.2929% COCA-COLA 0.2872% 0.2872% ABBVIE INC 0.2870% 0.2870% CHEVRON CORP 0.2767% 0.2767% WALMART INC 0.2767% -

Advz Md&A Q2 2020
SECOND QUARTER ENDED JUNE 30, 2020 MANAGEMENT’S DISCUSSION AND ANALYSIS August 12, 2020 Table of Contents 1 Management's Discussion and Analysis 2 2 Business Overview, Corporate Strategy and Segments 3 3 Recent Events 5 4 Results of Operations 8 5 Segment Performance 11 6 Corporate and Other Costs 14 7 Selected Quarterly Financial Information 17 8 Balance Sheet Analysis 19 9 Liquidity 21 10 Lending Arrangements and Debt 23 11 Contractual Obligations 24 12 Related Party Transactions 25 13 Non-IFRS Financial Measures 26 14 Critical Accounting Estimates 28 15 Contingencies 29 16 Outstanding Share Data 32 17 Control Environment 33 18 Forward-looking Statements 34 1 Management's Discussion and Analysis The following Management’s Discussion and Analysis ("MD&A") summarizes ADVANZ PHARMA Corp. Limited's (formerly known as ADVANZ PHARMA Corp.) (the "Company", "ADVANZ PHARMA", and together with its subsidiaries, the "Group", or "we" or "us" or "our") consolidated operating results and cash flows for the three and six month periods ended June 30, 2020 with the comparative prior periods, and the Company’s balance sheet as at June 30, 2020 and December 31, 2019. The MD&A was prepared as of August 12, 2020 and should be read in conjunction with the unaudited condensed interim consolidated financial statements and the notes thereto as at and for the three and six month periods ended June 30, 2020 and the consolidated financial statements and MD&A for the year ended December 31, 2019. Financial information in this MD&A is based on financial statements that have been prepared in accordance with International Financial Reporting Standards ("IFRS") as issued by the International Accounting Standards Board ("IASB") and amounts are stated in thousands of United States Dollars ("USD"), which is the reporting currency of the Company, unless otherwise noted.