Production, Use, Occurrence and Analysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Podiform Chromite Deposits—Database and Grade and Tonnage Models
Podiform Chromite Deposits—Database and Grade and Tonnage Models Scientific Investigations Report 2012–5157 U.S. Department of the Interior U.S. Geological Survey COVER View of the abandoned Chrome Concentrating Company mill, opened in 1917, near the No. 5 chromite mine in Del Puerto Canyon, Stanislaus County, California (USGS photograph by Dan Mosier, 1972). Insets show (upper right) specimen of massive chromite ore from the Pillikin mine, El Dorado County, California, and (lower left) specimen showing disseminated layers of chromite in dunite from the No. 5 mine, Stanislaus County, California (USGS photographs by Dan Mosier, 2012). Podiform Chromite Deposits—Database and Grade and Tonnage Models By Dan L. Mosier, Donald A. Singer, Barry C. Moring, and John P. Galloway Scientific Investigations Report 2012-5157 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior KEN SALAZAR, Secretary U.S. Geological Survey Marcia K. McNutt, Director U.S. Geological Survey, Reston, Virginia: 2012 This report and any updates to it are available online at: http://pubs.usgs.gov/sir/2012/5157/ For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment—visit http://www.usgs.gov or call 1–888–ASK–USGS For an overview of USGS information products, including maps, imagery, and publications, visit http://www.usgs.gov/pubprod To order this and other USGS information products, visit http://store.usgs.gov Suggested citation: Mosier, D.L., Singer, D.A., Moring, B.C., and Galloway, J.P., 2012, Podiform chromite deposits—database and grade and tonnage models: U.S. -

Sodium Chromate 10 Percent Section 1
Conforms to US OSHA Hazard Communication 29CFR1910.1200 SAFETY DATA SHEET Sodium Chromate 10 percent Section 1. Identification 1.1 Product identifier Product name : Sodium Chromate 10 percent Part no. : AR176, AR376 Validation date : 6/24/2020 1.2 Relevant identified uses of the substance or mixture and uses advised against Material uses : Laboratory use Container type: Dispenser Pack AR176 // Sodium Chromate 10 percent // Artisan Grocott's Methenamine Silver Stain Kit // 65 mL and 115 mL AR376 // Sodium Chromate 10 percent // Artisan Grocott's Methenamine Silver Eosin Stain Kit // 65 mL and 115 mL Reference number: SDS056 1.3 Details of the supplier of the safety data sheet Supplier/Manufacturer : Agilent Technologies, Inc. 5301 Stevens Creek Blvd Santa Clara, CA 95051, USA Tel: +1 800 227 9770 Agilent Technologies Singapore (International) Pte Ltd. No. 1 Yishun Avenue 7 Singapore, 768923 Tel. (65) 6276 2622 Agilent Technologies Denmark ApS Produktionsvej 42 2600 Glostrup, Denmark Tel. +45 44 85 95 00 www.Agilent.com e-mail address of person : [email protected] responsible for this SDS 1.4 Emergency telephone number In case of emergency : CHEMTREC®: 1-800-424-9300 Section 2. Hazards identification 2.1 Classification of the substance or mixture OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the substance or mixture Date of issue : 06/24/2020 1/15 Sodium Chromate 10 percent Section 2. Hazards identification H302 ACUTE TOXICITY (oral) - Category 4 H311 -

Material Safety Data Sheet
THE CHEM-MET COMPANY – SAFETY DATA SHEET – SODIUM MOLYBDATE DIHYDRATE THE CHEM-MET COMPANY 6419 YOCHELSON PLACE P.O. BOX 819 CLINTON, MARYLAND 20735 (301) 868-3355 FAX (301) 868-3355 SAFETY DATA SHEET SODIUM MOLYBDATE DIHYDRATE PRODUCT INFORMATION DATA SHEET SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1. Product identifier. Substance name: Sodium Molybdate Dihydrate. Chemical formula: Na2MoO4 · 2 H2O. Synonyms/trade names: Sodium Molybdate Dehydrate. Disodium Molybdate Dihydrate. Sodium Molybdate Crystals Index No. (Regulation (EC) No 1272/2008): None. CAS Numbers: 10102-40-6. 7631-95-0 (for the anhydrous Na2MoO4). EC No.: 231-551-7 (assumed to cover both dihydrate and anhydrous form). 1.2. Relevant identified uses of the substance or mixture and uses advised against: 1.2.1. Relevant identified uses: Micronutrient in manufacture and use of fertilizers. Micronutrient in feed additives. Corrosion inhibitor. Manufacture of pigments. Industrial detergent for metal surface treatment. Cleaning & maintenance material. As coolant/anti-freeze/heat transfer fluid. Metal working fluids. Industrial formulation & use of lubrication additives, lubricants and greases. Manufacture of enamels frits, ceramics. Manufacture & use of water treatments chemicals, inc. water softener. Polymer preparations & compounds. Industrial chemical products such as pH regulator, flocculants, precipitants, neutralization agents Extraction agents. Photochemicals. Manufacture and use of catalysts, inc. regeneration & recycling. 1.2.2. Uses advised against: There are no identified uses advised against. 1.3. Details of the supplier of the safety data sheet: THE CHEM-MET COMPANY 6419 YOCHELSON PLACE/ P.O. BOX 819 CLINTON, MARYLAND 20735 USA Tel: 301.868.3355 Fax: 301.868.8946 1 Email: [email protected] (Victor Fox, President); [email protected] (Brendan Hickey, Vice President) THE CHEM-MET COMPANY – SAFETY DATA SHEET – SODIUM MOLYBDATE DIHYDRATE 1.4. -

Chromite Crystal Structure and Chemistry Applied As an Exploration Tool
Western University Scholarship@Western Electronic Thesis and Dissertation Repository February 2015 Chromite Crystal Structure and Chemistry applied as an Exploration Tool Patrick H.M. Shepherd The University of Western Ontario Supervisor Dr. Roberta L. Flemming The University of Western Ontario Graduate Program in Geology A thesis submitted in partial fulfillment of the equirr ements for the degree in Master of Science © Patrick H.M. Shepherd 2015 Follow this and additional works at: https://ir.lib.uwo.ca/etd Part of the Geology Commons Recommended Citation Shepherd, Patrick H.M., "Chromite Crystal Structure and Chemistry applied as an Exploration Tool" (2015). Electronic Thesis and Dissertation Repository. 2685. https://ir.lib.uwo.ca/etd/2685 This Dissertation/Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. Western University Scholarship@Western University of Western Ontario - Electronic Thesis and Dissertation Repository Chromite Crystal Structure and Chemistry Applied as an Exploration Tool Patrick H.M. Shepherd Supervisor Roberta Flemming The University of Western Ontario Follow this and additional works at: http://ir.lib.uwo.ca/etd Part of the Geology Commons This Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in University of Western Ontario - Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. Chromite Crystal Structure and Chemistry Applied as an Exploration Tool (Thesis format: Integrated Article) by Patrick H.M. -

Wood Modified by Inorganic Salts: Mechanism and Properties
WOOD MODIFIED BY INORGANIC SALTS: MECHANISM AND PROPERTIES. I. WEATHERING RATE, WATER REPELLENCY, AND IIIMENSIONAL STABILITY OF WOOD MODIFIEID WITH CHROMIUM (111) NITRATE VERSUS CHROMIC ACID' R. S. Willianzs and W. C. Feisl Research Chemists Forest Products Lab~ratory,~Forest Service U.S. Department of Agriculture Madison, WI 53705 (Received October 1983) ABSTRACT Chromic acid treatment of wood improves surface characteristics. A simple dip or brush application of 5% aqueous chromic acid to a wood surface stops extractive staining, improves dimensional stability, retards weathering of unfinished wood, and prolongs the life of finishes. A better understanding of how chromic acid effects these improvements may facilitate the development of even better treatments. The improvements may be due to a combination offactors: oxidation ofwood by hexavalent chromium compounds, formation of coordination coml~lexeswith trivalent chromium, and the presence of insoluble chromium compounds at the surface. Most trivalent chromium compounds do not become fixed in wood (i.e., do not form water-insolublf: wood-chemical complexes). However, chromium (111) nitrate treatment of western redcedar (Thuja plicata), southern pine (Pinus sp.), and ponderosa pine (Pinus ponderosa) produced modified wood with properties similar to chromic acid-treated wood. Evaluation of the chromium (111) nitrate- and chromic acid-modified wood by leaching, Xenon arc- accelerated weathering, and swellometer experiments clearly demonstrated similar fixation, erosion, and water-repellent properties. Based on the results from chromium (111) nitrate-treated wood, fixation through coordination of trivalent chromium with wood hydroxyls an(5 formation of insoluble chromium appear to be the critical factors in improving the performance of the treated wood. -

(VI) and Chromium (V) Oxide Fluorides
Portland State University PDXScholar Dissertations and Theses Dissertations and Theses 1976 The chemistry of chromium (VI) and chromium (V) oxide fluorides Patrick Jay Green Portland State University Follow this and additional works at: https://pdxscholar.library.pdx.edu/open_access_etds Part of the Chemistry Commons Let us know how access to this document benefits ou.y Recommended Citation Green, Patrick Jay, "The chemistry of chromium (VI) and chromium (V) oxide fluorides" (1976). Dissertations and Theses. Paper 4039. https://doi.org/10.15760/etd.5923 This Thesis is brought to you for free and open access. It has been accepted for inclusion in Dissertations and Theses by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. All ABSTRACT OF THE TllESIS OF Patrick Jay Green for the Master of Science in Chemistry presented April 16, 1976. Title: Chemistry of Chromium(VI) and Chromium(V) Oxide Fluorides. APPROVEO BY MEMBERS OF THE THESIS CO'"o\l TIEE: y . • Ii . ' I : • • • • • New preparative routes to chromyl fluoride were sought. It was found that chlorine ironofluoride reacts with chromium trioxide and chromyl chlo ride to produce chromyl fluoride. Attempts were ~ade to define a mechan ism for the reaction of ClF and Cr0 in light of by-products observed 3 and previous investigations. Carbonyl fluoride and chromium trioxide react to fom chro·yl fluoride and carbo:i dioxide. A mechanism was also proposed for this react10n. Chromium trioxide 11itl\ l~F6 or WF5 reacts to produce chromyl fluoride and the respective oxide tetrafluoride. 2 Sulfur hexafluoride did not react with Cr03. -

Chromium Compounds Hazard Summary
Chromium Compounds Hazard Summary Chromium occurs in the environment primarily in two valence states, trivalent chromium (Cr III) and hexavalent chromium (Cr VI). Exposure may occur from natural or industrial sources of chromium. Chromium III is much less toxic than chromium (VI). The respiratory tract is also the major target organ for chromium (III) toxicity, similar to chromium (VI). Chromium (III) is an essential element in humans. The body can detoxify some amount of chromium (VI) to chromium (III). The respiratory tract is the major target organ for chromium (VI) toxicity, for acute (short-term) and chronic (long-term) inhalation exposures. Shortness of breath, coughing, and wheezing were reported from a case of acute exposure to chromium (VI), while perforations and ulcerations of the septum, bronchitis, decreased pulmonary function, pneumonia, and other respiratory effects have been noted from chronic exposure. Human studies have clearly established that inhaled chromium (VI) is a human carcinogen, resulting in an increased risk of lung cancer. Animal studies have shown chromium (VI) to cause lung tumors via inhalation exposure. Please Note: The main sources of information for this fact sheet are EPA's Integrated Risk Information System (IRIS) (7), which contains information on inhalation chronic toxicity and the RfC and oral chronic toxicity and the RfD, and the carcinogenic effects of chromium including the unit cancer risk for inhalation exposure, EPA's Toxicological Review of Trivalent Chromium and Toxicological Review of Hexavalent Chromium (3), and the Agency for Toxic Substances and Disease Registry's (ATSDR's) Toxicological Profile for Chromium. (1) Uses The metal chromium is used mainly for making steel and other alloys. -

Category Approaches, Read-Across, (Q)SAR Technical Report No
Category approaches, Read-across, (Q)SAR Technical Report No. 116 EUROPEAN CENTRE FOR ECOTOXICOLOGY AND TOXICOLOGY OF CHEMICALS Category approaches, Read-across, (Q)SAR Technical Report No. 116 Brussels, November 2012 ISSN-0773-8072-116 (print) ISSN-2079-1526-116 (online) Category approaches, Read-across, (Q)SAR ECETOC Technical Report No. 116 © Copyright – ECETOC AISBL European Centre for Ecotoxicology and Toxicology of Chemicals 2 Avenue E. Van Nieuwenhuyse (Bte 8), B-1160 Brussels, Belgium. All rights reserved. No part of this publication may be reproduced, copied, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise without the prior written permission of the copyright holder. Applications to reproduce, store, copy or translate should be made to the Secretary General. ECETOC welcomes such applications. Reference to the document, its title and summary may be copied or abstracted in data retrieval systems without subsequent reference. The content of this document has been prepared and reviewed by experts on behalf of ECETOC with all possible care and from the available scientific information. It is provided for information only. ECETOC cannot accept any responsibility or liability and does not provide a warranty for any use or interpretation of the material contained in the publication. ECETOC TR No. 116 Category approaches, Read-across, (Q)SAR Category approaches, Read-across, (Q)SAR TABLE OF CONTENTS SUMMARY 1 1. INTRODUCTION 3 1.1 Terms of reference 4 1.2 Scope 5 1.3 Roadmap of the report 6 2. DEFINITIONS FOR NON-TESTING APPROACHES 8 2.1 Data gap filling 9 2.1.1 Read-across 9 2.1.2 Trend analysis and computational methods based on internal models 13 2.1.3 External (Q)SAR models and expert systems 14 3. -

Chemical Resistance Chart
CHEMICAL RESISTANCE CHART CHEMICAL RESISTANCE DATA These recommendations are based upon information from material suppliers and careful examination of available published information and are believed to be accurate. However, since the resistance of metals, plastics and elastomers can be affected by concentration, temperature, presence of other chemicals and other factors. This information should be considered as a general guide rather than an unqualified guarantee. Ultimately, the customer must determine the suitability of the pump used in various solutions. All recommendations assume ambient temperatures unless otherwise noted. RATINGS — CHEMICAL EFFECT FOOTNOTES A — No effect—Excellent 1. P.V.C. — Satisfactory to 72 °F B — Minor effect—Good 2. Polypropylene — Satisfactory to 72 °F C — Moderate effect—Fair 3. Polypropylene — Satisfactory to 120 °F D — Severe effect—Not recommended 4. Nitrile — Satisfactory for “O” Rings 5. Polyacetal — Satisfactory to 72 °F 6. Ceramag — Satisfactory to 72 °F The ratings for these materials are based upon the chemical resistance only. Added consideration must be given to pump selections when the chemical is abrasive, viscous in nature, or has a Specific Gravity greater than 1.1. NOTE: The materials shown below in BOLDFACE TYPE are used in the construction of Little Giant chemical pumps. ” 302 Stainless Steel 304 Stainless Steel 316 Stainless Steel 440 Stainless Steel Aluminum TITANIUM C276 NICKEL ALLOY Cast Bronze Brass Cast Iron Carbon Steel 1) PVC (Type (E-3606) Tygon PTFE Polyacetal Nylon ABS Thermoplastic -

Spinel Group Minerals in Metamorphosed Ultramafic Rocks from Río De Las Tunas Belt, Central Andes, Argentina
Geologica Acta, Vol.11, Nº 2, June 2013, 133-148 DOI: 10.1344/105.000001836 Available online at www.geologica-acta.com Spinel group minerals in metamorphosed ultramafic rocks from Río de Las Tunas belt, Central Andes, Argentina 1 1 2 M.F. GARGIULO E.A. BJERG A. MOGESSIE 1 INGEOSUR (Universidad Nacional del Sur – CONICET) San Juan 670, B8000ICN Bahía Blanca, Argentina Gargiulo E-mail: [email protected]; [email protected] Bjerg E-mail: [email protected] 2 Institut für Erdwissenschaften, Bereich Mineralogie und Petrologie, Karl-Franzens Universität Graz Universitätsplatz 2, 8010 Graz, Austria E-mail: [email protected] ABS TRACT In the Río de Las Tunas belt, Central Andes of Argentina, spinel group minerals occur in metaperidotites and in reaction zones developed at the boundary between metaperidotite bodies and their country-rocks. They comprise two types: i) Reddish-brown crystals with compositional zonation characterized by a ferritchromite core surrounded by an inner rim of Cr-magnetite and an outer rim of almost pure magnetite. ii) Green crystals chemically homogeneous with spinel (s.s.) and/or pleonaste compositions. The mineral paragenesis Fo+Srp+Cln+Tr+Fe-Chr and Fo+Cln+Tr+Tlc±Ath+Fe-Chr observed in the samples indicate lower and middle grade amphibolite facies metamorphic conditions. Nonetheless, the paragenesis (green)Spl+En+Fo±Di indicates that granulite facies conditions were also reached at a few localities. Cr-magnetite and magnetite rims in zoned reddish-brown crystals and magnetite rims around green-spinel/pleonaste grains are attributed to a later serpentinization process during retrograde metamorphism. -

Chemical Compatibility Guide
Chemical Compatibility Guide Guide Applicable to the Following: PIG Portable Spill Containment Pool Guide Information This report is offered as a guide and was developed from information which, to the best of New Pig’s knowledge, was reliable and accurate. Due to variables and conditions of application beyond New Pig’s control, none of the data shown in this guide is to be construed as a guarantee, expressed or implied. New Pig assumes no responsibility, obligation, or liability in conjunction with the use or misuse of the information. PIG Spill Containment Pools are constructed from PVC-coated polyester fabric. The chemical resistance guide that follows shows the chemical resistance for the PVC layer only. This guide has been compiled to provide the user with general chemical resistance information. It does not reflect actual product testing. Ratings / Key or Ratings – Chemical Effect 1. Satisfactory to 72°F (22°C) 2. Satisfactory to 120°F (48°C) A = Excellent D = Severe Effect, not recommended for ANY use. B = Good — Minor Effect, slight corrosion or discoloration. N/A = Information not available. C = Fair — Moderate Effect, not recommended for continuous use. Softening, loss of strength, swelling may occur. Due to variables and conditions beyond our control, New Pig cannot guarantee that this product(s) will work to your satisfaction. To ensure effectiveness and your safety, we recommend that you conduct compatibility and absorption testing of your chemicals with this product prior to purchase. For additional questions or information, -
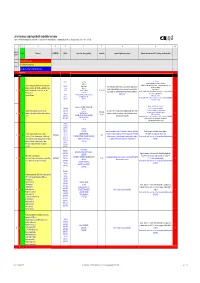
List of Substances Subject to Prohibited / Undesirable / Declaration
List of substances subject to prohibited / undesirable / declaration Appendix 1 to the Guidelines to Design and Purchasing Prohibition of Harmful Substances : ebmpapstMulfingen: 90-001 ebmpapstSt.Georgen: WN 3-12.2 ebmpapstLandshut: 60118.00040 (Ver. 5.0-31.07.2008) 1 2 3 4 5 6 7 8 9 10 Consecu N - new Substance EU-INDEX-No.: CAS-No. Source (law, decree, guideline). Hazard/risk example of application/occurrence Remarks and comments (of 0.1% deviating consideration limit) tive No. V = prohibited (verboten) U = undesirable (unerwünscht) D = subject to declaration (deklarationspflichtig) V = prohibited RoHS from 100 ppm 7439-92-1 TRGS 905, Pb-stabilisers and pigments are subject to declaration. GefStoffV, Prohibited: Anhydrite neutral lead carbonate, lead hydrogen carbonate and lead Lead and compounds (red lead oxide, lead sulphate, lead (7446-14-2 ChemVerbotsV, cable coating, heat transfer medium for accumulators, hybrid integrated sulphate as dye pigment hydrogen carbonate, lead carbonate, lead phtalates, lead 1319-46-6 BatterieV, circuits, stabilisers in plastics, vessels and pipes for aggressive liquids, < 0.4 % in batteries 1 V N acetates, lead phosphates, lead stereates , etc) lead 598-63-0 2002/95/EG (RoHS), K3, R 1, R 3 V E F according to RoHS: Lead as alloying additive in aluminum (up to 0.4 mass%) in steel (up to 67/458/EEC pigment production, lead alloys, anti-corrosion agents (fuel additives), chromate: refer to 0.35 mass%), in copper (up to 4%), 301-04-2 76/769/EEC (89/667/EEC, 97/10/EC, 97/56/EC) soldering agent chromium (VI)