WO 2017/213252 Al 14 December 2017 (14.12.2017) W !P O PCT
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Redalyc.Catalogue of the Family Sesiidae in China
SHILAP Revista de Lepidopterología ISSN: 0300-5267 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España Jin, Q.; Wang, S. X.; Li, H. H. Catalogue of the family Sesiidae in China (Lepidoptera: Sesiidae) SHILAP Revista de Lepidopterología, vol. 36, núm. 144, diciembre, 2008, pp. 507-526 Sociedad Hispano-Luso-Americana de Lepidopterología Madrid, España Available in: http://www.redalyc.org/articulo.oa?id=45511220017 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative 507-526 Catalogue of the family 10/12/08 10:40 Página 507 SHILAP Revta. lepid., 36 (144), diciembre 2008: 507-526 CODEN: SRLPEF ISSN:0300-5267 Catalogue of the family Sesiidae in China (Lepidoptera: Sesiidae) Q. Jin, S. X. Wang & H. H. Li Abstract A catalogue of the family Sesiidae in China is provided based partially on the research of the previous literature and partially on the study of the specimens in our collection. A total of 108 species in 26 genera are listed, along with the available information of distribution and host plants. KEY WORDS: Lepidoptera, Sesiidae, catalogue, host plants, distribution, China. Catálogo de la familia Sesiidae en China (Lepidoptera: Sesiidae) Resumen Se presenta un catálogo de la familia Sesiidae en China basado parcialmente sobre las revisiones bibliográficas y parcialmente sobre el estudio de los especímenes en nuestra colección. Se da una lista de 108 especies en 26 géneros, así como la información disponible de su distribución y plantas nutricias. -

Health and Sustainable Management of Teak Stands
Health and sustainable management of teak stands V.V. Sudheendrakumar Kerala Forest Research Institute Peechi- 680653, Thrissur, Kerala, India Email: [email protected] Abstract Teak ( Tectona grandis L.f.) has been planted in over 36 countries across the tropical and subtropical regions in the Asian, African and American continents as well as many islands in the Pacific and Atlantic oceans. The insect pests of teak can be broadly classified as defoliators, stem borers and root feeders. Defoliators cause loss in volume increment in plantations as they feed on leaves. The most important defoliators are the lepidopterans namely, Hyblaea puera , Eutectona machaeralis and Paliga damastesalis . The borers belonging to the Lepidoptera include Alcterogystia cadambae , Xyleutes ceramicus and Sahydrassus malabaricus . The root feeders include white grubs belonging to the coleopterans. For most of the above pests data is not available on the economic impact caused except the information on the damage potential. H. puera is the only pest for which economic loss has been assessed based on the work done in Kerala. Accordingly effective biocontrol strategies involving a baculovirus have been developed and field- tested to manage this pest. However, no routine defoliator management is being practiced in any teak growing areas. Disease incidences are common in nurseries and plantations. Major diseases encountered are bacterial collar rot, bacterial wilt, pink disease, Phomopsis leaf spot, Colletotrichum leaf spot, leaf rust, etc. Chemical control is usually adopted for the management of such diseases. Keywords: Insect pests of teak , defoliators, Hyblaea puera, stem borers, biocontrol, diseases of teak Introduction Teak ( Tectona grandis L.f.) whose natural distribution was limited originally to some parts of South and Southeast Asia, is now one of the most widespread tropical tree species. -

The Sphingidae (Lepidoptera) of the Philippines
©Entomologischer Verein Apollo e.V. Frankfurt am Main; download unter www.zobodat.at Nachr. entomol. Ver. Apollo, Suppl. 17: 17-132 (1998) 17 The Sphingidae (Lepidoptera) of the Philippines Willem H o g e n e s and Colin G. T r e a d a w a y Willem Hogenes, Zoologisch Museum Amsterdam, Afd. Entomologie, Plantage Middenlaan 64, NL-1018 DH Amsterdam, The Netherlands Colin G. T readaway, Entomologie II, Forschungsinstitut Senckenberg, Senckenberganlage 25, D-60325 Frankfurt am Main, Germany Abstract: This publication covers all Sphingidae known from the Philippines at this time in the form of an annotated checklist. (A concise checklist of the species can be found in Table 4, page 120.) Distribution maps are included as well as 18 colour plates covering all but one species. Where no specimens of a particular spe cies from the Philippines were available to us, illustrations are given of specimens from outside the Philippines. In total we have listed 117 species (with 5 additional subspecies where more than one subspecies of a species exists in the Philippines). Four tables are provided: 1) a breakdown of the number of species and endemic species/subspecies for each subfamily, tribe and genus of Philippine Sphingidae; 2) an evaluation of the number of species as well as endemic species/subspecies per island for the nine largest islands of the Philippines plus one small island group for comparison; 3) an evaluation of the Sphingidae endemicity for each of Vane-Wright’s (1990) faunal regions. From these tables it can be readily deduced that the highest species counts can be encountered on the islands of Palawan (73 species), Luzon (72), Mindanao, Leyte and Negros (62 each). -

Konzept Für Lokalfauna
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Mitteilungen der Entomologischen Arbeitsgemeinschaft Salzkammergut Jahr/Year: 2004 Band/Volume: 2004 Autor(en)/Author(s): Kallies Axel, Pühringer Franz Artikel/Article: Provisional checklist of the Sesiidae of the world (Lepidoptera: Ditrysia) 1-85 ©Salzkammergut Entomologenrunde; download unter www.biologiezentrum.at Mitt.Ent.Arb.gem.Salzkammergut 4 1-85 4.12.2004 Provisional checklist of the Sesiidae of the world (Lepidoptera: Ditrysia) Franz PÜHRINGER & Axel KALLIES Abstract: A checklist of Sesiidae of the world provides 2453 names, 1562 of which are currently considered valid taxa (1 family, 2 subfamilies, 10 tribes, 149 genera, 1352 species, and 48 subspecies). Data concerning distribution, type species or type genus, designation, incorrect spelling and emendation, preoccupation and replacement names, synonyms and homonyms, nomina nuda, and rejected names are given. Several new combinations and synonyms are provided. Key words: Sesioidea, systematics, taxonomy, zoogeographic regions. Introduction: Almost 25 years have passed since HEPPNER & DUCKWORTH (1981) published their 'Classification of the Superfamily Sesioidea'. In the meantime great progress has been made in the investigation and classification of the family Sesiidae (clearwing moths). Important monographs covering the Palearctic and Nearctic regions, and partly South America or South-East Asia have been made available (EICHLIN & DUCKWORTH 1988, EICHLIN 1986, 1989, 1995b and 1998, ŠPATENKA et al. 1999, KALLIES & ARITA 2004), and numerous descriptions of new taxa as well as revisions of genera and species described by earlier authors have been published, mainly dealing with the Oriental region (ARITA & GORBUNOV 1995c, ARITA & GORBUNOV 1996b etc.). -

27 Skunk Vine
27 SKUNK VINE R. W. Pemberton and P. D. Pratt U.S. Department of Agriculture, Agricultural Research Service, Invasive Plant Research Laboratory, Fort Lauderdale, Florida, USA tion of livestock, however, are unknown (Gann and PEST STATUS OF WEED Gordon, 1998). In urban landscapes, this vine en- Skunk vine, Paederia foetida L. (Fig. 1), is a recently twines branches of woody ornamental plants and also recognized weedy vine of natural areas in Florida that spreads horizontally through lawns, rooting at the is spreading into other parts of the southern United nodes (Martin, 1995). In westcentral Florida, P. States. The weed, which is native to Asia, appears to foetida is considered the most troublesome weed have the potential to spread well beyond the South along roadside right-of-ways (W. Moriaty, pers. to the northeastern states. Control of the plant by comm.), and it also entangles power lines and associ- chemical or mechanical means damages valued veg- ated structures (Martin, 1995). etation supporting the vine. Skunk vine is a Category On the island of Hawaii, P. foetida is a very se- I Florida Exotic Pest Plant Council weed (Langeland rious weed in nurseries producing ornamental foli- and Craddock Burks, 1998), a listing that groups the age plants (Pemberton, pers. obs.). The weed infests plant with the most invasive weed species in Florida. field plantings used for propagation. Control of the weed is very difficult because stock plants are easily injured if herbicides are applied. At times, growers have had to abandon or destroy stock plants that have become overgrown by skunk vine. -

Diptera: Tachinidae: Exoristinae) from Iran, with New Records
J. Agr. Sci. Tech. (2021) Vol. 23(5): 1073-1090 Review of the Tribe Eryciini Robineau-Desvoidy (Diptera: Tachinidae: Exoristinae) from Iran, with New Records F. Seyyedi-Sahebari1*, S. Khaghaninia2, and A. A. Talebi 3 ABSTRACT The present paper contains a review of the tribe Eryciini (Tachinidae: Exoristinae) that occur in Iran. Twenty-six species belonging to 15 genera are reviewed. The collected data on 20 species are provided. Of these, seven genera and 16 species are newly recorded. Distribution and host information are briefly summarized. The diagnostic characters of the new records species are given. The key to the species found in Iran is also provided. Keywords: Taxonomy, Fauna of Eryciini, Diptera, Parasitoid flies. INTRODUCTION at most with 1-3 setae; first postsutural supra-alar setae longer and stronger than the Tachinidae is the second largest dipteran notopleural setae and first postsutural intra- family with more than 8,500 described alar seta, middorsal depression on species, distributed worldwide (O’Hara et abdominal syntergite 1+2 usually extending al., 2020). The larvae are endoparasitoids of to posterior margin of that segment; true other arthropods, mainly insects. Exoristinae binding membrane between sternites 6-7 in represent one of the largest subfamilies in males is wide; sensilla trichodea on sternite the Tachinidae, containing seven tribes in 5 existing in most genera (Tschorsnig and the Palearctic Region. Eryciini is a large and Herting, 1994). diverse tribe with wide distribution in the Although the history of investigation on world (Herting, 1984; O’Hara and Wood, tachinid fauna of Iran is not very long, there 2004). -

Part III. Pests of Selected Forest Tree Species
PART III Pests of selected forest tree species PART III Pests of selected forest tree species 143 Abies grandis Order and Family: Pinales: Pinaceae Common names: grand fir; giant fir NATURAL DISTRIBUTION Abies grandis is a western North American (both Pacific and Cordilleran) species (Klinka et al., 1999). It grows in coastal (maritime) and interior (continental) regions from latitude 39 to 51 °N and at a longitude of 125 to 114 °W. In coastal regions, it grows in southern British Columbia (Canada), in the interior valleys and lowlands of western Washington and Oregon (United States), and in northwestern California (United States). Its range extends to eastern Washington, northern Idaho, western Montana, and northeastern Oregon (Foiles, 1965; Little, 1979). This species is not cultivated as an exotic to any significant extent. PESTS Arthropods in indigenous range The western spruce budworm (Choristoneura occidentalis) and Douglas-fir tussock moth (Orgyia pseudotsugata) have caused widespread defoliation, top kill and mortality to grand fir. Early-instar larvae of C. occidentalis mine and kill the buds, while late- instar larvae are voracious and wasteful feeders, often consuming only parts of needles, chewing them off at their bases. The western balsam bark beetle (Dryocoetes confusus) and the fir engraver (Scolytus ventralis) are the principal bark beetles. Fir cone moths (Barbara spp.), fir cone maggots (Earomyia spp.), and several seed chalcids destroy large numbers of grand fir cones and seeds. The balsam woolly adelgid (Adelges piceae) is a serious pest of A. grandis in western Oregon, Washington and southwestern British Columbia (Furniss and Carolin, 1977). Feeding by this aphid causes twigs to swell or ‘gout’ at the nodes and the cambium produces wide, irregular annual growth rings consisting of reddish, highly lignified, brittle wood (Harris, 1978). -

The Major Arthropod Pests and Weeds of Agriculture in Southeast Asia
The Major Arthropod Pests and Weeds of Agriculture in Southeast Asia: Distribution, Importance and Origin D.F. Waterhouse (ACIAR Consultant in Plant Protection) ACIAR (Australian Centre for International Agricultural Research) Canberra AUSTRALIA The Australian Centre for International Agricultural Research (ACIAR) was established in June 1982 by an Act of the Australian Parliament. Its mandate is to help identify agricultural problems in developing countries and to commission collaborative research between Australian and developing country researchers in fields where Australia has a special research competence. Where trade names are used this constitutes neither endorsement of nor discrimination against any product by the Centre. ACIAR MO'lOGRAPH SERIES This peer-reviewed series contains the results of original research supported by ACIAR, or deemed relevant to ACIAR's research objectives. The series is distributed internationally, with an emphasis on the Third World. © Australian Centre for 1I1lernational Agricultural Resl GPO Box 1571, Canberra, ACT, 2601 Waterhouse, D.F. 1993. The Major Arthropod Pests an Importance and Origin. Monograph No. 21, vi + 141pI- ISBN 1 86320077 0 Typeset by: Ms A. Ankers Publication Services Unit CSIRO Division of Entomology Canberra ACT Printed by Brown Prior Anderson, 5 Evans Street, Burwood, Victoria 3125 ii Contents Foreword v 1. Abstract 2. Introduction 3 3. Contributors 5 4. Results 9 Tables 1. Major arthropod pests in Southeast Asia 10 2. The distribution and importance of major arthropod pests in Southeast Asia 27 3. The distribution and importance of the most important arthropod pests in Southeast Asia 40 4. Aggregated ratings for the most important arthropod pests 45 5. Origin of the arthropod pests scoring 5 + (or more) or, at least +++ in one country or ++ in two countries 49 6. -

Macro Moths of Tinsukia District, Assam: a JEZS 2017; 5(6): 1612-1621 © 2017 JEZS Provisional Inventory Received: 10-09-2017 Accepted: 11-10-2017
Journal of Entomology and Zoology Studies 2017; 5(6): 1612-1621 E-ISSN: 2320-7078 P-ISSN: 2349-6800 Macro moths of Tinsukia district, Assam: A JEZS 2017; 5(6): 1612-1621 © 2017 JEZS provisional inventory Received: 10-09-2017 Accepted: 11-10-2017 Subhasish Arandhara Subhasish Arandhara, Suman Barman, Rubul Tanti and Abhijit Boruah Upor Ubon Village, Kakopather, Tinsukia, Assam, India Abstract Suman Barman This list reports 333 macro moth species for the Tinsukia district of Assam, India. The moths were Department of Wildlife Sciences, captured by light trapping as well as by opportunistic sighting across 37 sites in the district for a period of Gauhati University, Assam, three years from 2013-2016. Identification was based on material and visual examination of the samples India with relevant literature and online databases. The list includes the family, subfamily, tribes, scientific name, the author and year of publication of description for each identified species. 60 species in this Rubul Tanti inventory remain confirmed up to genus. Department of Wildlife Biology, A.V.C. College, Tamil Nadu, Keywords: Macro moths, inventory, Lepidoptera, Tinsukia, Assam India Introduction Abhijit Boruah Upor Ubon Village, Kakopather, The order Lepidoptera, a major group of plant-eating insects and thus, from the agricultural Tinsukia, Assam, India and forestry point of view they are of immense importance [1]. About 134 families comprising 157, 000 species of living Lepidoptera, including the butterflies has been documented globally [2], holding around 17% of the world's known insect fauna. Estimates, however, suggest more species in the order [3]. Naturalists for convenience categorised moths into two informal groups, the macro moths having larger physical size and recency in evolution and micro moths [4] that are smaller in size and primitive in origin . -

Appendix 9: Insects Sub-Group Report HONG KONG TERRESTRIAL AND
Status, Trends and Red Listing – Terrestrial and Fresh Water Insects Sub Group Report – August 2014 1 Appendix 9: Insects Sub-group Report HONG KONG TERRESTRIAL AND FRESHWATER INSECTS STATUS, TRENDS, RED LISTING & RECOMMENDATIONS AUGUST 2014 EXECUTIVE SUMMARY Hong Kong insect biodiversity is at present poorly known, despite the fact that this is the most diverse group of multicellular organisms on the planet. Our knowledge of the status of the local insect fauna is limited to a few insect taxonomic groups (butterflies & moths, dragonflies & damselflies, mayflies, caddisflies, ants, partially beetles, partially aculeate wasp taxa)and in consequence only a few insect taxa have been assessed locally. Knowledge of population trends is minimal. However, over 2400 species of moth, 240 butterflies, 115 dragonflies & damselflies, 180 ants, 70 mosquitoes, 40 termites, and over 150 aculeate Wasps have been recorded locally, as well as hundreds of beetles and smaller numbers of mayflies, caddisflies and stick insects. Some of these insects are endemic to Hong Kong and many species either entirely new to science or to the territory are discovered every year. An assessment conducted by the authors on a fraction of these Hong Kong species has revealed that 29 out of 46 moths , eight out of 10 aculeate wasps and three out of 104 dragonflies assessed qualified for threatened status (Critically Endangered, Endangered and Vulnerable) as defined by IUCN. The ecology of these insects is diverse and understanding this is important to conserving the biota as a whole. A particular need is to study those that perform important known ecological services such as pollination, seed dispersal, nutrient recycling and soil turnover, and to gain a better understanding of their functional role within food webs. -
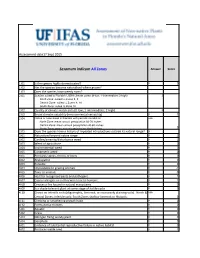
WRA.Datasheet.Template
Assessment&date17&Sept&2015 Sesamum'indicum*All*Zones* Answer Score 1.01 Is&the&species&highly&domesticated? n 0 1.02 Has&the&species&become&naturalised&where&grown? 1.03 Does&the&species&have&weedy&races? 2.01 Species suited to Florida's USDA climate zones (0-low; 1-intermediate; 2-high) 2 North Zone: suited to Zones 8, 9 Central Zone: suited to Zones 9, 10 South Zone: suited to Zone 10 2.02 Quality&of&climate&match&data&(0Blow;&1Bintermediate;&2Bhigh) 2 2.03 Broad&climate&suitability&(environmental&versatility) y 1 2.04 Native or naturalized in habitats with periodic inundation unk North Zone: mean annual precipitation 50-70 inches Central Zone: mean annual precipitation 40-60 inches South Zone: mean annual precipitation 40-60 inches 0 2.05 Does&the&species&have&a&history&of&repeated&introductions&outside&its&natural&range? y 3.01 Naturalized&beyond&native&range y 2 3.02 Garden/amenity/disturbance&weed y 2 3.03 Weed&of&agriculture n 0 3.04 Environmental&weed n 0 3.05 Congeneric&weed n 0 4.01 Produces&spines,þs&or&burrs n 0 4.02 Allelopathic n 0 4.03 Parasitic n 0 4.04 Unpalatable&to&grazing&animals n -1 4.05 Toxic&to&animals n 0 4.06 Host&for&recognised&pests&and&pathogens y 1 4.07 Causes&allergies&or&is&otherwise&toxic&to&humans y 1 4.08 Creates&a&fire&hazard&in&natural&ecosystems n 0 4.09 Is&a&shade&tolerant&plant&at&some&stage&of&its&life&cycle n 0 4.10 Grows&on&infertile&soils&(oligotrophic,&limerock,&or&excessively&draining&soils).&&North&&& unk Central&Zones:&infertile&soils;&South&Zone:&shallow&limerock&or&Histisols. -

Nepal, with Area 147181 Km
CHAPTER I INTRODUCTION 1.1 Background Nepal, with area 147,181 km2, occupies the central part of Himalaya that stands between the Palaeartic (Holartic) and Plaeotropical (Indo-Malayan) regions. The country is located between latitudes 26o22' and 30o27' N and longitudes 80o40' and 88o12' E. The country is partitioned lengthwise into Palearctic and Oriental sets of floral and faunal provinces (Smith, 1989). Nepal comprises only 0.09% of land area on a global scale, but it possesses a disproportionately rich diversity of flora and fauna at genetic, species and ecosystem levels. Nepal has a relatively high number of fauna species invertebrates and vertebrates both. Higher fauna groups have been relatively well studied, however the taxonomy and distribution of the lower fauna groups, except for the butterflies and to some extent the spiders, have yet to be studied. An inventory made by Thapa (1997) covers approximately 5,052 species of insects recorded from Nepal, 1,131 species (over 22 percent) have been first discovered and described from Nepalese specimen. The entomological inventory recorded 789 species of moths and 656 species of butterflies (Thapa, 1998). Among Nepal‟s insect fauna, many taxonomists have worked on butterflies of Nepal and a fair amount of taxonomic studies and identification guides are available for the group (Smith 1989, 1990). 640 species of butterflies have been recorded, distributed in different ecological zones. Smith's book is the first to cover this extremely interesting fauna in a comprehensive format. He has given the complete species and subspecies name, author, date of publication, common name if available, range of wingspan, comments on distribution (usually to district within Nepal), seasonality, elevational range, distribution outside Nepal, and the species' relative abundance.