Health and Sustainable Management of Teak Stands
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WO 2017/213252 Al 14 December 2017 (14.12.2017) W !P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2017/213252 Al 14 December 2017 (14.12.2017) W !P O PCT (51) International Patent Classification: TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, C07D 413/04 (2006.01) C07D 271/07 (2006.01) EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, LV, C07D 417/10 (2006.01) A01N 43/836 (2006.01) MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, SM, TR), OAPI (BF, BJ, CF, CG, CI, CM, GA, GN, GQ, GW, (21) International Application Number: KM, ML, MR, NE, SN, TD, TG). PCT/JP20 17/02 1473 (22) International Filing Date: Published: 09 June 2017 (09.06.2017) — with international search report (Art. 21(3)) (25) Filing Language: English (26) Publication Language: English (30) Priority Data: 2016-1 15977 10 June 2016 (10.06.2016) 2016-239161 09 December 2016 (09.12.2016) (71) Applicant: SUMITOMO CHEMICAL COMPANY, LIMITED [JP/JP]; 27-1, Shinkawa 2-chome, Chuo-ku, Tokyo, 1048260 (JP). (72) Inventors: ARIMORI, Sadayuki; c/o SUMITOMO CHEMICAL COMPANY, LIMITED, 2-1, Takatsukasa 4-chome, Takarazuka-shi, Hyogo, 6658555 (JP). YA- MAMOTO, Masaoki; c/o SUMITOMO CHEMICAL COMPANY, LIMITED, 2-1, Takatsukasa 4-chome, Takarazuka-shi, Hyogo, 6658555 (JP). (74) Agent: SAMEJIMA, Mutsumi et al; AOYAMA & PARTNERS, Umeda Hankyu Bldg. Office Tower, 8-1, Kakuda-cho, Kita-ku, Osaka-shi, Osaka, 5300017 (JP). (81) Designated States (unless otherwise indicated, -

The Sphingidae (Lepidoptera) of the Philippines
©Entomologischer Verein Apollo e.V. Frankfurt am Main; download unter www.zobodat.at Nachr. entomol. Ver. Apollo, Suppl. 17: 17-132 (1998) 17 The Sphingidae (Lepidoptera) of the Philippines Willem H o g e n e s and Colin G. T r e a d a w a y Willem Hogenes, Zoologisch Museum Amsterdam, Afd. Entomologie, Plantage Middenlaan 64, NL-1018 DH Amsterdam, The Netherlands Colin G. T readaway, Entomologie II, Forschungsinstitut Senckenberg, Senckenberganlage 25, D-60325 Frankfurt am Main, Germany Abstract: This publication covers all Sphingidae known from the Philippines at this time in the form of an annotated checklist. (A concise checklist of the species can be found in Table 4, page 120.) Distribution maps are included as well as 18 colour plates covering all but one species. Where no specimens of a particular spe cies from the Philippines were available to us, illustrations are given of specimens from outside the Philippines. In total we have listed 117 species (with 5 additional subspecies where more than one subspecies of a species exists in the Philippines). Four tables are provided: 1) a breakdown of the number of species and endemic species/subspecies for each subfamily, tribe and genus of Philippine Sphingidae; 2) an evaluation of the number of species as well as endemic species/subspecies per island for the nine largest islands of the Philippines plus one small island group for comparison; 3) an evaluation of the Sphingidae endemicity for each of Vane-Wright’s (1990) faunal regions. From these tables it can be readily deduced that the highest species counts can be encountered on the islands of Palawan (73 species), Luzon (72), Mindanao, Leyte and Negros (62 each). -

Diptera: Tachinidae: Exoristinae) from Iran, with New Records
J. Agr. Sci. Tech. (2021) Vol. 23(5): 1073-1090 Review of the Tribe Eryciini Robineau-Desvoidy (Diptera: Tachinidae: Exoristinae) from Iran, with New Records F. Seyyedi-Sahebari1*, S. Khaghaninia2, and A. A. Talebi 3 ABSTRACT The present paper contains a review of the tribe Eryciini (Tachinidae: Exoristinae) that occur in Iran. Twenty-six species belonging to 15 genera are reviewed. The collected data on 20 species are provided. Of these, seven genera and 16 species are newly recorded. Distribution and host information are briefly summarized. The diagnostic characters of the new records species are given. The key to the species found in Iran is also provided. Keywords: Taxonomy, Fauna of Eryciini, Diptera, Parasitoid flies. INTRODUCTION at most with 1-3 setae; first postsutural supra-alar setae longer and stronger than the Tachinidae is the second largest dipteran notopleural setae and first postsutural intra- family with more than 8,500 described alar seta, middorsal depression on species, distributed worldwide (O’Hara et abdominal syntergite 1+2 usually extending al., 2020). The larvae are endoparasitoids of to posterior margin of that segment; true other arthropods, mainly insects. Exoristinae binding membrane between sternites 6-7 in represent one of the largest subfamilies in males is wide; sensilla trichodea on sternite the Tachinidae, containing seven tribes in 5 existing in most genera (Tschorsnig and the Palearctic Region. Eryciini is a large and Herting, 1994). diverse tribe with wide distribution in the Although the history of investigation on world (Herting, 1984; O’Hara and Wood, tachinid fauna of Iran is not very long, there 2004). -

Part III. Pests of Selected Forest Tree Species
PART III Pests of selected forest tree species PART III Pests of selected forest tree species 143 Abies grandis Order and Family: Pinales: Pinaceae Common names: grand fir; giant fir NATURAL DISTRIBUTION Abies grandis is a western North American (both Pacific and Cordilleran) species (Klinka et al., 1999). It grows in coastal (maritime) and interior (continental) regions from latitude 39 to 51 °N and at a longitude of 125 to 114 °W. In coastal regions, it grows in southern British Columbia (Canada), in the interior valleys and lowlands of western Washington and Oregon (United States), and in northwestern California (United States). Its range extends to eastern Washington, northern Idaho, western Montana, and northeastern Oregon (Foiles, 1965; Little, 1979). This species is not cultivated as an exotic to any significant extent. PESTS Arthropods in indigenous range The western spruce budworm (Choristoneura occidentalis) and Douglas-fir tussock moth (Orgyia pseudotsugata) have caused widespread defoliation, top kill and mortality to grand fir. Early-instar larvae of C. occidentalis mine and kill the buds, while late- instar larvae are voracious and wasteful feeders, often consuming only parts of needles, chewing them off at their bases. The western balsam bark beetle (Dryocoetes confusus) and the fir engraver (Scolytus ventralis) are the principal bark beetles. Fir cone moths (Barbara spp.), fir cone maggots (Earomyia spp.), and several seed chalcids destroy large numbers of grand fir cones and seeds. The balsam woolly adelgid (Adelges piceae) is a serious pest of A. grandis in western Oregon, Washington and southwestern British Columbia (Furniss and Carolin, 1977). Feeding by this aphid causes twigs to swell or ‘gout’ at the nodes and the cambium produces wide, irregular annual growth rings consisting of reddish, highly lignified, brittle wood (Harris, 1978). -

The Major Arthropod Pests and Weeds of Agriculture in Southeast Asia
The Major Arthropod Pests and Weeds of Agriculture in Southeast Asia: Distribution, Importance and Origin D.F. Waterhouse (ACIAR Consultant in Plant Protection) ACIAR (Australian Centre for International Agricultural Research) Canberra AUSTRALIA The Australian Centre for International Agricultural Research (ACIAR) was established in June 1982 by an Act of the Australian Parliament. Its mandate is to help identify agricultural problems in developing countries and to commission collaborative research between Australian and developing country researchers in fields where Australia has a special research competence. Where trade names are used this constitutes neither endorsement of nor discrimination against any product by the Centre. ACIAR MO'lOGRAPH SERIES This peer-reviewed series contains the results of original research supported by ACIAR, or deemed relevant to ACIAR's research objectives. The series is distributed internationally, with an emphasis on the Third World. © Australian Centre for 1I1lernational Agricultural Resl GPO Box 1571, Canberra, ACT, 2601 Waterhouse, D.F. 1993. The Major Arthropod Pests an Importance and Origin. Monograph No. 21, vi + 141pI- ISBN 1 86320077 0 Typeset by: Ms A. Ankers Publication Services Unit CSIRO Division of Entomology Canberra ACT Printed by Brown Prior Anderson, 5 Evans Street, Burwood, Victoria 3125 ii Contents Foreword v 1. Abstract 2. Introduction 3 3. Contributors 5 4. Results 9 Tables 1. Major arthropod pests in Southeast Asia 10 2. The distribution and importance of major arthropod pests in Southeast Asia 27 3. The distribution and importance of the most important arthropod pests in Southeast Asia 40 4. Aggregated ratings for the most important arthropod pests 45 5. Origin of the arthropod pests scoring 5 + (or more) or, at least +++ in one country or ++ in two countries 49 6. -

Macro Moths of Tinsukia District, Assam: a JEZS 2017; 5(6): 1612-1621 © 2017 JEZS Provisional Inventory Received: 10-09-2017 Accepted: 11-10-2017
Journal of Entomology and Zoology Studies 2017; 5(6): 1612-1621 E-ISSN: 2320-7078 P-ISSN: 2349-6800 Macro moths of Tinsukia district, Assam: A JEZS 2017; 5(6): 1612-1621 © 2017 JEZS provisional inventory Received: 10-09-2017 Accepted: 11-10-2017 Subhasish Arandhara Subhasish Arandhara, Suman Barman, Rubul Tanti and Abhijit Boruah Upor Ubon Village, Kakopather, Tinsukia, Assam, India Abstract Suman Barman This list reports 333 macro moth species for the Tinsukia district of Assam, India. The moths were Department of Wildlife Sciences, captured by light trapping as well as by opportunistic sighting across 37 sites in the district for a period of Gauhati University, Assam, three years from 2013-2016. Identification was based on material and visual examination of the samples India with relevant literature and online databases. The list includes the family, subfamily, tribes, scientific name, the author and year of publication of description for each identified species. 60 species in this Rubul Tanti inventory remain confirmed up to genus. Department of Wildlife Biology, A.V.C. College, Tamil Nadu, Keywords: Macro moths, inventory, Lepidoptera, Tinsukia, Assam India Introduction Abhijit Boruah Upor Ubon Village, Kakopather, The order Lepidoptera, a major group of plant-eating insects and thus, from the agricultural Tinsukia, Assam, India and forestry point of view they are of immense importance [1]. About 134 families comprising 157, 000 species of living Lepidoptera, including the butterflies has been documented globally [2], holding around 17% of the world's known insect fauna. Estimates, however, suggest more species in the order [3]. Naturalists for convenience categorised moths into two informal groups, the macro moths having larger physical size and recency in evolution and micro moths [4] that are smaller in size and primitive in origin . -
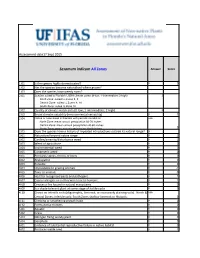
WRA.Datasheet.Template
Assessment&date17&Sept&2015 Sesamum'indicum*All*Zones* Answer Score 1.01 Is&the&species&highly&domesticated? n 0 1.02 Has&the&species&become&naturalised&where&grown? 1.03 Does&the&species&have&weedy&races? 2.01 Species suited to Florida's USDA climate zones (0-low; 1-intermediate; 2-high) 2 North Zone: suited to Zones 8, 9 Central Zone: suited to Zones 9, 10 South Zone: suited to Zone 10 2.02 Quality&of&climate&match&data&(0Blow;&1Bintermediate;&2Bhigh) 2 2.03 Broad&climate&suitability&(environmental&versatility) y 1 2.04 Native or naturalized in habitats with periodic inundation unk North Zone: mean annual precipitation 50-70 inches Central Zone: mean annual precipitation 40-60 inches South Zone: mean annual precipitation 40-60 inches 0 2.05 Does&the&species&have&a&history&of&repeated&introductions&outside&its&natural&range? y 3.01 Naturalized&beyond&native&range y 2 3.02 Garden/amenity/disturbance&weed y 2 3.03 Weed&of&agriculture n 0 3.04 Environmental&weed n 0 3.05 Congeneric&weed n 0 4.01 Produces&spines,þs&or&burrs n 0 4.02 Allelopathic n 0 4.03 Parasitic n 0 4.04 Unpalatable&to&grazing&animals n -1 4.05 Toxic&to&animals n 0 4.06 Host&for&recognised&pests&and&pathogens y 1 4.07 Causes&allergies&or&is&otherwise&toxic&to&humans y 1 4.08 Creates&a&fire&hazard&in&natural&ecosystems n 0 4.09 Is&a&shade&tolerant&plant&at&some&stage&of&its&life&cycle n 0 4.10 Grows&on&infertile&soils&(oligotrophic,&limerock,&or&excessively&draining&soils).&&North&&& unk Central&Zones:&infertile&soils;&South&Zone:&shallow&limerock&or&Histisols. -

Nepal, with Area 147181 Km
CHAPTER I INTRODUCTION 1.1 Background Nepal, with area 147,181 km2, occupies the central part of Himalaya that stands between the Palaeartic (Holartic) and Plaeotropical (Indo-Malayan) regions. The country is located between latitudes 26o22' and 30o27' N and longitudes 80o40' and 88o12' E. The country is partitioned lengthwise into Palearctic and Oriental sets of floral and faunal provinces (Smith, 1989). Nepal comprises only 0.09% of land area on a global scale, but it possesses a disproportionately rich diversity of flora and fauna at genetic, species and ecosystem levels. Nepal has a relatively high number of fauna species invertebrates and vertebrates both. Higher fauna groups have been relatively well studied, however the taxonomy and distribution of the lower fauna groups, except for the butterflies and to some extent the spiders, have yet to be studied. An inventory made by Thapa (1997) covers approximately 5,052 species of insects recorded from Nepal, 1,131 species (over 22 percent) have been first discovered and described from Nepalese specimen. The entomological inventory recorded 789 species of moths and 656 species of butterflies (Thapa, 1998). Among Nepal‟s insect fauna, many taxonomists have worked on butterflies of Nepal and a fair amount of taxonomic studies and identification guides are available for the group (Smith 1989, 1990). 640 species of butterflies have been recorded, distributed in different ecological zones. Smith's book is the first to cover this extremely interesting fauna in a comprehensive format. He has given the complete species and subspecies name, author, date of publication, common name if available, range of wingspan, comments on distribution (usually to district within Nepal), seasonality, elevational range, distribution outside Nepal, and the species' relative abundance. -

Insect Environment Quarterly Journal
Volume 24 (2) (June) 2021 ISSN 0975-1963 Insect Environment Quarterly journal IE is abstracted in CABI and ZooBank An atmanirbhar iniave by Indian entomologists for promong Insect Science Published by International Phytosanitary Research & Services For Private Circulation only Editorial Board Editor-in-Chief Dr. Jose Romeno Faleiro, Former FAO Expert, IPM Dr. Abraham Verghese Specialist (Red Palm Weevil), Middle East and South Former Director, ICAR-National Bureau of Asia Agricultural Insect Resources (NBAIR), Bangalore, Former Principal Scientist & Head Entomology, ICAR- Prof. Dr. Abdeljelil Bakri, Former Head of the Insect Indian Institute of Horticultural Research, Bengaluru, Biological Control Unit at Cadi Ayyad University- Former Chief Editor, Pest Management in Horticultural Marrakech, Morocco. FAO and IAEA Consultant, Ecosystem Editor of Fruit Fly News e-newsletter, Canada Co-Editor-in-Chief Dr. Hamadttu Abdel Farag El-Shafie (Ph.D), Senior Dr. Rashmi, M.A, Senior Technical Officer Research Entomologist, Head, Sustainable pest (Entomology), Regional Plant Quarantine Station, management in date palm research program , Date Bengaluru Palm Research Center of Excellence (DPRC) , King Editors Faisal University, B.O. 55031, Al-Ahsa 31982, Saudi Arabia Dr. Devi Thangam. S, Assistant Professor Zoology, MES College, Bengaluru Dr. B. Vasantharaj David, Trustee, Secretary & Treasurer, Dr. B. Vasantharaj David Foundation, Dr. Badal Bhattacharyya, Principal Scientist, Chennai Department of Entomology, Assam Agricultural University, Jorhat, Assam Dr. V.V. Ramamurthy, Editorial Advisor, Indian Journal of Entomology, Former Principal Scientist & Dr. Viyolla Pavana Mendonce, Assistant Professor Head Entomology, IARI, Pusa Campus, New Delhi Zoology, School of Life Sciences, St. Joseph’s College (Autonomous), Bengaluru Rev. Dr. S. Maria Packiam, S.J, Director, Entomology Research Institute (ERI), Loyola College, Dr. -

& 1. Actias Selene (Hubner) 2. Acherontia Lachesis (Fabricius) 3
Ree. %001. Surv. India, 86(2) : 399-403, 1990 SHORT COMMUNICATIONS NEW RECORDS OF LEPIDOPTERA FROM ARUNACHAL PRADESH, INDIA The present communication is based on the study of material collected from Arunachal Pradesh by Mr. C. B. Prasad, Museum and Taxidermy section of Zoological Suvey of India, Calcutta. As a result of this study 15 species in 15 genera and nine families belonging to suborder Heterocera have been recorded for the first time from Arunachal Pradesh. Betts (1950), Mandai & Ghosh (1967), Varshney & Chanda (1971), Arora & Chaudhury (1976), Gupta & Shukla (1977a,b), Arora & MandaI (1981), Arora & Chaudhury (1980, 1982) and Bhattacharya (1985) have reported earlier a large number of species of butterflies and moths from Arunachal Pradesh. With new locality records reported in this note, the distribution of the included species has been extended. Family SATURNllDAE 1. Actias selene (Hubner) Material examined: 1 cf, Arunachal Pradesh, Indira Park, 24.iv.1987. Wing expanse: 93 mm. Distribution : India (Sikkim, Arunachal Pradesh, Assam, Manipur, Meghalaya, Bihar, West Bengal, Orissa, Tamil nadu, Karnataka, Maharashtra, Uttar Pradesh, Himachal Pradesh and Andamans); Nepal; Bhutan; Burma; Bangladesh; Sri Lanka; Borneo; Tibet; USSR; and Japan. Remark : The wing expanse of the examined material is much shorter than hitherto recorded (132 - 166 mm.). Family SPHINGIDAE 2. Acherontia lachesis (Fabricius) Material examined: 1 9, Arunachal Pradesh, Rajbhawan, 26.iv.1987. Wing expanse: 118 mm. Distribution : India (Sikkim, Arunachal pradesh, Assam, Meghalaya, West Bengal, Orissa, Maharashtra and Andaman Islands); Bangladesh; Sri Lanka; Java; and Hongkong. 3. Theretra nessus (Drury) Material examined : 1 9, Arunachal Pradesh, Chief Minister's Bungalow, 27.iv.1987. -

20 Years of S’ PRINT JOURNAL & Journal F Threatened Taxa (April 1999–March 2019) ISSN 0974-7907 (Online); ISSN 0974-7893 (Print)
ISSN 0974-7907 (Online) ISSN 0974-7893 (Print) Journal of Threatened Taxa 26 March 2019 (Online & Print) Vol. 11 | No. 5 | 13511–13630 PLATINUM 10.11609/jott.2019.11.5.13511-13630 OPEN www.threatenedtaxa.org ACCESS J Building TTevidence for conservation globally 20 years of S’ PRINT JOURNAL & Journal f Threatened Taxa (April 1999–March 2019) ISSN 0974-7907 (Online); ISSN 0974-7893 (Print) Publisher Host Wildlife Information Liaison Development Society Zoo Outreach Organization www.wild.zooreach.org www.zooreach.org No. 12, Thiruvannamalai Nagar, Saravanampatti - Kalapatti Road, Saravanampatti, Coimbatore, Tamil Nadu 641035, India Ph: +91 9385339863 | www.threatenedtaxa.org Email: [email protected] EDITORS Typesetting Founder & Chief Editor Mr. Arul Jagadish, ZOO, Coimbatore, India Dr. Sanjay Molur Mrs. Radhika, ZOO, Coimbatore, India Wildlife Information Liaison Development (WILD) Society & Zoo Outreach Organization (ZOO), Mrs. Geetha, ZOO, Coimbatore India 12 Thiruvannamalai Nagar, Saravanampatti, Coimbatore, Tamil Nadu 641035, India Mr. Ravindran, ZOO, Coimbatore India Deputy Chief Editor Fundraising/Communications Dr. Neelesh Dahanukar Mrs. Payal B. Molur, Coimbatore, India Indian Institute of Science Education and Research (IISER), Pune, Maharashtra, India Editors/Reviewers Managing Editor Subject Editors 2016-2018 Mr. B. Ravichandran, WILD, Coimbatore, India Fungi Associate Editors Dr. B.A. Daniel, ZOO, Coimbatore, Tamil Nadu 641035, India Dr. B. Shivaraju, Bengaluru, Karnataka, India Ms. Priyanka Iyer, ZOO, Coimbatore, Tamil Nadu 641035, India Prof. Richard Kiprono Mibey, Vice Chancellor, Moi University, Eldoret, Kenya Dr. Mandar Paingankar, Department of Zoology, Government Science College Gadchiroli, Dr. R.K. Verma, Tropical Forest Research Institute, Jabalpur, India Chamorshi Road, Gadchiroli, Maharashtra 442605, India Dr. V.B. Hosagoudar, Bilagi, Bagalkot, India Dr. -

The Macroecology of Southeast-Asian Hawkmoths (Lepidoptera: Sphingidae)
The macroecology of Southeast-Asian hawkmoths (Lepidoptera: Sphingidae) Dissertation zur Erlangung des naturwissenschaftlichen Doktorgrades der Bayerischen Julius-Maximiliams-Universität Würzburg vorgelegt von Jan Beck aus Bamberg Würzburg, 2005 Eingereicht am: 21. Januar 2005 Mitglieder der Promotionskommission: Vorsitzender: Prof. Dr. Ulrich Scheer Gutachter: Prof. Dr. K. Eduard Linsenmair Gutachter: Prof. Dr. Konrad Fiedler Tag des Promotionskolloquiums: ……………………………………….. Doktorurkunde ausgehändigt am: …………………………………………… Table of contents Preface Page 1 Chapter 1 – Introduction 3 1.1 The concept of large-scaled ecological research 3 1.2 Study region and study taxon 5 1.3 Retrieving and processing information for macroecological research 9 Colour plates Chapter 2 – Feasibility of light trapping 13 Chapter 3 – Local Assemblages 35 3.1 Local species diversity 35 3.2 Rank-abundance distributions 55 Chapter 4 – Regional Assemblages 67 4.1 Species richness and biogeography 67 4.2 Range size measurements 93 Chapter 5 – Niche dimensions 105 5.1 Larval host spectrum and dietary breadth 105 5.2 The distribution of body sizes 119 Chapter 6 – Range-Abundance relationships 131 Chapter 7 – Synthesis & General Discussion 161 Summaries 165 Deutsche Zusammenfassung 165 English Summary 169 Ringkasan dalam Bahasa Malaysia 173 Ringkasan dalam Bahasa Indonesia 177 References 181 Acknowledgements 205 Curriculum Vitae 207 Ehrenwörtliche Erklärung 209 Appendix 211 I) Quantitative sampling sites 211 II) New records from own sampling 213 On CD-ROM: Website ‘The