Infrared Spectroscopy (Ir)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Radiant Heating with Infrared
W A T L O W RADIANT HEATING WITH INFRARED A TECHNICAL GUIDE TO UNDERSTANDING AND APPLYING INFRARED HEATERS Bleed Contents Topic Page The Advantages of Radiant Heat . 1 The Theory of Radiant Heat Transfer . 2 Problem Solving . 14 Controlling Radiant Heaters . 25 Tips On Oven Design . 29 Watlow RAYMAX® Heater Specifications . 34 The purpose of this technical guide is to assist customers in their oven design process, not to put Watlow in the position of designing (and guaranteeing) radiant ovens. The final responsibility for an oven design must remain with the equipment builder. This technical guide will provide you with an understanding of infrared radiant heating theory and application principles. It also contains examples and formulas used in determining specifications for a radiant heating application. To further understand electric heating principles, thermal system dynamics, infrared temperature sensing, temperature control and power control, the following information is also available from Watlow: • Watlow Product Catalog • Watlow Application Guide • Watlow Infrared Technical Guide to Understanding and Applying Infrared Temperature Sensors • Infrared Technical Letter #5-Emissivity Table • Radiant Technical Letter #11-Energy Uniformity of a Radiant Panel © Watlow Electric Manufacturing Company, 1997 The Advantages of Radiant Heat Electric radiant heat has many benefits over the alternative heating methods of conduction and convection: • Non-Contact Heating Radiant heaters have the ability to heat a product without physically contacting it. This can be advantageous when the product must be heated while in motion or when physical contact would contaminate or mar the product’s surface finish. • Fast Response Low thermal inertia of an infrared radiation heating system eliminates the need for long pre-heat cycles. -

5G Candidate Band Study
5G Candidate Band Study Study on the Suitability of Potential Candidate Frequency Bands above 6GHz for Future 5G Mobile Broadband Systems Final Report to Ofcom, March 2015 Contributors Steve Methley, William Webb, Stuart Walker, John Parker Quotient Associates Limited Compass House, Vision Park, Chivers Way, Histon, Cambridge, CB24 9AD, UK EMAIL [email protected] WEB www.QuotientAssociates.com Ver si on 02 Status Approved History Reviewed by Ofcom Type Public 5G Candidate Band Study | Contributors Final Report : qa1015 © Quotient Associates Ltd. 2015 Commercial in Confidence. No part of the contents of this document may be disclosed, used or reproduced in any form, or by any means, without the prior written consent of Quotient Associates Ltd. ii EXECUTIVE SUMMARY Objective and scope Within industry and academia there is significant activity in research and development towards the next generation of mobile broadband technologies (5G). Coupled with this there is increasing interest in identifying the frequency bands that will be needed to deliver 5G services. At the moment there is no overall consensus on what 5G will actually be. Work in ITU-R (WP5D) is ongoing to develop a vision for 5G (IMT 2020) including identifying the target capabilities. It is, however, widely accepted that at least one element of 5G will require the use of spectrum bands at much higher frequencies than those that current mobile broadband technologies can make use of, e.g. bands above 6 GHz. A first step currently being discussed is a proposal for an agenda item for the World Radio Conference after next (WRC-19) to identify suitable high frequency spectrum for 5G in the ITU Radio Regulations. -

The Origin of the Peculiarities of the Vietnamese Alphabet André-Georges Haudricourt
The origin of the peculiarities of the Vietnamese alphabet André-Georges Haudricourt To cite this version: André-Georges Haudricourt. The origin of the peculiarities of the Vietnamese alphabet. Mon-Khmer Studies, 2010, 39, pp.89-104. halshs-00918824v2 HAL Id: halshs-00918824 https://halshs.archives-ouvertes.fr/halshs-00918824v2 Submitted on 17 Dec 2013 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Published in Mon-Khmer Studies 39. 89–104 (2010). The origin of the peculiarities of the Vietnamese alphabet by André-Georges Haudricourt Translated by Alexis Michaud, LACITO-CNRS, France Originally published as: L’origine des particularités de l’alphabet vietnamien, Dân Việt Nam 3:61-68, 1949. Translator’s foreword André-Georges Haudricourt’s contribution to Southeast Asian studies is internationally acknowledged, witness the Haudricourt Festschrift (Suriya, Thomas and Suwilai 1985). However, many of Haudricourt’s works are not yet available to the English-reading public. A volume of the most important papers by André-Georges Haudricourt, translated by an international team of specialists, is currently in preparation. Its aim is to share with the English- speaking academic community Haudricourt’s seminal publications, many of which address issues in Southeast Asian languages, linguistics and social anthropology. -
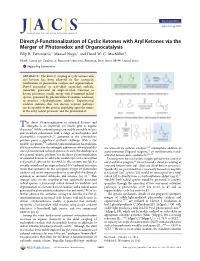
Direct Β‑Functionalization of Cyclic Ketones with Aryl Ketones Via the Merger of Photoredox and Organocatalysis Filip R
Communication pubs.acs.org/JACS Direct β‑Functionalization of Cyclic Ketones with Aryl Ketones via the Merger of Photoredox and Organocatalysis Filip R. Petronijevic,́† Manuel Nappi,† and David W. C. MacMillan* Merck Center for Catalysis at Princeton University, Princeton, New Jersey 08544, United States *S Supporting Information ABSTRACT: The direct β-coupling of cyclic ketones with aryl ketones has been achieved via the synergistic combination of photoredox catalysis and organocatalysis. Diaryl oxymethyl or aryl−alkyl oxymethyl radicals, transiently generated via single-electron reduction of ketone precursors, readily merge with β-enaminyl radical species, generated by photon-induced enamine oxidation, to produce γ-hydroxyketone adducts. Experimental evidence indicates that two discrete reaction pathways can be operable in this process depending upon the nature of the ketyl radical precursor and the photocatalyst. he direct β-functionalization of saturated ketones and T aldehydes is an important yet elusive goal in organic chemistry.1 While carbonyl groups are readily amenable to ipso- and α-carbon substitution with a range of nucleophiles and electrophiles respectively,2,3 activation at the β-methylene position poses a significant synthetic challenge. With a few notable exceptions,1,4 carbonyl β-functionalization has tradition- ally been restricted to the conjugate addition of soft nucleophiles are accessed via carbene catalysis,9,10 nucleophilic addition of into α,β-unsaturated carbonyl systems. As such, the development acetal-protected Grignard reagents,11 or stoichiometric metal- 5 − of a general catalytic platform for the direct β-functionalization activated homoenolate equivalents.12 16 of saturated ketones or aldehydes would represent a conceptual Drawing from the mechanistic insights gained in the course of and practical advance for the field. -
Understanding Infrared Light
TEACHER/PARENT ACTIVITY MANUAL Joint Polar Satellite System Understanding Infrared Light This activity educates students about the electromagnetic spectrum, or different forms of light detected by Earth observing satellites. The Joint Polar Satellite System (JPSS), a collaborative effort between NOAA and NASA, detects various wavelengths of the electromagnetic spectrum including infrared light to measure the temperature of Earth’s surface, oceans, and atmosphere. The data from these measurements provide the nation with accurate weather forecasts, hurricane warnings, wildfire locations, and much more! Provided is a list of materials that can be purchased to complete several learning activities, including simulating infrared light by constructing homemade infrared goggles. Learning Objectives Next Generation Science Standards (Grades 5–8) Performance Disciplinary Description Expectation Core Ideas 4-PS4-1 PS4.A: • Waves, which are regular patterns of motion, can be made in water Waves and Their Wave Properties by disturbing the surface. When waves move across the surface of Applications in deep water, the water goes up and down in place; there is no net Technologies for motion in the direction of the wave except when the water meets a Information Transfer beach. (Note: This grade band endpoint was moved from K–2.) • Waves of the same type can differ in amplitude (height of the wave) and wavelength (spacing between wave peaks). 4-PS4-2 PS4.B: An object can be seen when light reflected from its surface enters the Waves and Their Electromagnetic eyes. Applications in Radiation Technologies for Information Transfer 4-PS3-2 PS3.B: Light also transfers energy from place to place. -

The Reaction of Aminonitriles with Aminothiols: a Way to Thiol-Containing Peptides and Nitrogen Heterocycles in the Primitive Earth Ocean
life Article The Reaction of Aminonitriles with Aminothiols: A Way to Thiol-Containing Peptides and Nitrogen Heterocycles in the Primitive Earth Ocean Ibrahim Shalayel , Seydou Coulibaly, Kieu Dung Ly, Anne Milet and Yannick Vallée * Univ. Grenoble Alpes, CNRS, Département de Chimie Moléculaire, Campus, F-38058 Grenoble, France; [email protected] (I.S.); [email protected] (S.C.); [email protected] (K.D.L.); [email protected] (A.M.) * Correspondence: [email protected] Received: 28 September 2018; Accepted: 18 October 2018; Published: 19 October 2018 Abstract: The Strecker reaction of aldehydes with ammonia and hydrogen cyanide first leads to α-aminonitriles, which are then hydrolyzed to α-amino acids. However, before reacting with water, these aminonitriles can be trapped by aminothiols, such as cysteine or homocysteine, to give 5- or 6-membered ring heterocycles, which in turn are hydrolyzed to dipeptides. We propose that this two-step process enabled the formation of thiol-containing dipeptides in the primitive ocean. These small peptides are able to promote the formation of other peptide bonds and of heterocyclic molecules. Theoretical calculations support our experimental results. They predict that α-aminonitriles should be more reactive than other nitriles, and that imidazoles should be formed from transiently formed amidinonitriles. Overall, this set of reactions delineates a possible early stage of the development of organic chemistry, hence of life, on Earth dominated by nitriles and thiol-rich peptides (TRP). Keywords: origin of life; prebiotic chemistry; thiol-rich peptides; cysteine; aminonitriles; imidazoles 1. Introduction In ribosomes, peptide bonds are formed by the reaction of the amine group of an amino acid with an ester function. -

Aldehydes and Ketones
12 Aldehydes and Ketones Ethanol from alcoholic beverages is first metabolized to acetaldehyde before being broken down further in the body. The reactivity of the carbonyl group of acetaldehyde allows it to bind to proteins in the body, the products of which lead to tissue damage and organ disease. Inset: A model of acetaldehyde. (Novastock/ Stock Connection/Glow Images) KEY QUESTIONS 12.1 What Are Aldehydes and Ketones? 12.8 What Is Keto–Enol Tautomerism? 12.2 How Are Aldehydes and Ketones Named? 12.9 How Are Aldehydes and Ketones Oxidized? 12.3 What Are the Physical Properties of Aldehydes 12.10 How Are Aldehydes and Ketones Reduced? and Ketones? 12.4 What Is the Most Common Reaction Theme of HOW TO Aldehydes and Ketones? 12.1 How to Predict the Product of a Grignard Reaction 12.5 What Are Grignard Reagents, and How Do They 12.2 How to Determine the Reactants Used to React with Aldehydes and Ketones? Synthesize a Hemiacetal or Acetal 12.6 What Are Hemiacetals and Acetals? 12.7 How Do Aldehydes and Ketones React with CHEMICAL CONNECTIONS Ammonia and Amines? 12A A Green Synthesis of Adipic Acid IN THIS AND several of the following chapters, we study the physical and chemical properties of compounds containing the carbonyl group, C O. Because this group is the functional group of aldehydes, ketones, and carboxylic acids and their derivatives, it is one of the most important functional groups in organic chemistry and in the chemistry of biological systems. The chemical properties of the carbonyl group are straightforward, and an understanding of its characteristic reaction themes leads very quickly to an understanding of a wide variety of organic reactions. -

Atomic and Molecular Laser-Induced Breakdown Spectroscopy of Selected Pharmaceuticals
Article Atomic and Molecular Laser-Induced Breakdown Spectroscopy of Selected Pharmaceuticals Pravin Kumar Tiwari 1,2, Nilesh Kumar Rai 3, Rohit Kumar 3, Christian G. Parigger 4 and Awadhesh Kumar Rai 2,* 1 Institute for Plasma Research, Gandhinagar, Gujarat-382428, India 2 Laser Spectroscopy Research Laboratory, Department of Physics, University of Allahabad, Prayagraj-211002, India 3 CMP Degree College, Department of Physics, University of Allahabad, Pragyagraj-211002, India 4 Physics and Astronomy Department, University of Tennessee, University of Tennessee Space Institute, Center for Laser Applications, 411 B.H. Goethert Parkway, Tullahoma, TN 37388-9700, USA * Correspondence: [email protected]; Tel.: +91-532-2460993 Received: 10 June 2019; Accepted: 10 July 2019; Published: 19 July 2019 Abstract: Laser-induced breakdown spectroscopy (LIBS) of pharmaceutical drugs that contain paracetamol was investigated in air and argon atmospheres. The characteristic neutral and ionic spectral lines of various elements and molecular signatures of CN violet and C2 Swan band systems were observed. The relative hardness of all drug samples was measured as well. Principal component analysis, a multivariate method, was applied in the data analysis for demarcation purposes of the drug samples. The CN violet and C2 Swan spectral radiances were investigated for evaluation of a possible correlation of the chemical and molecular structures of the pharmaceuticals. Complementary Raman and Fourier-transform-infrared spectroscopies were used to record the molecular spectra of the drug samples. The application of the above techniques for drug screening are important for the identification and mitigation of drugs that contain additives that may cause adverse side-effects. Keywords: paracetamol; laser-induced breakdown spectroscopy; cyanide; carbon swan bands; principal component analysis; Raman spectroscopy; Fourier-transform-infrared spectroscopy 1. -

Using Visible and Near-Infrared Reflectance Spectroscopy and Differential Scanning Calorimetry to Study Starch, Protein, and Temperature Effects on Bread Staling
Using Visible and Near-Infrared Reflectance Spectroscopy and Differential Scanning Calorimetry to Study Starch, Protein, and Temperature Effects on Bread Staling Feng Xie,1 Floyd E. Dowell,2,3 and Xiuzhi S. Sun1 ABSTRACT Cereal Chem. 81(2):249–254 Starch, protein, and temperature effects on bread staling were inves- retrograded amylose-lipid complex was limited. Two important wave- tigated using visible and near-infrared spectroscopy (NIRS) and differ- lengths, 550 nm and 1,465 nm, were key for NIRS to successfully classify ential scanning calorimetry (DSC). Bread staling was mainly due to the starch-starch (SS) and starch-protein (SP) bread based on different amylopectin retrogradation. NIRS measured amylopectin retrogradation colors and protein contents in SS and SP. Low temperature dramatically accurately in different batches. Three important wavelengths, 970 nm, accelerated the amylopectin retrogradation process. Protein retarded bread 1,155 nm, and 1,395 nm, were associated with amylopectin retrogra- staling, but not as much as temperature. The starch and protein interaction dation. NIRS followed moisture and starch structure changes when was less important than the starch retrogradation. Protein hindered the amylopectin retrograded. The amylose-lipid complex changed little from bread staling process mainly by diluting starch and retarding starch retro- one day after baking. The capability of NIRS to measure changes in the gradation. Bread staling is a complex process that occurs during bread and starch (Osborne and Douglas 1981; Davies and Miller 1988; storage. It is a progressive deterioration of qualities such as taste, Millar et al 1996). Therefore, NIRS has the potential to provide fun- firmness, etc. -

Hydrogen Atom Transfer-Mediated Cyclisations of Nitriles
Hydrogen Atom Transfer-Mediated Cyclisations of Nitriles Oliver J. Turner,*[a,b] John A. Murphy,*[b] David. J. Hirst[a] and Eric P. A. Talbot*[a]† Abstract: Hydrogen atom transfer-mediated intramolecular C-C coupling reactions between alkenes and nitriles, using PhSiH3 and catalytic Fe(acac)3, are described. This introduces a new strategic bond disconnection for ring-closing reactions, forming ketones via imine intermediates. Of note is the scope of the reaction, including formation of sterically hindered ketones, spirocycles and fused cyclic systems. In the early 1960s, Kwiatek and Seyler first reported the use of metal hydrides as catalysts in the hydrogenation of α,β- unsaturated compounds.[1,2] The discovery by Halpern,[3] later elegantly developed by Norton,[4] that metal-hydride hydrogen atom transfer (HAT) proceeded by a free-radical mechanism opened the door to a wide range of alkene hydrofunctionalisation reactions. But it was the pioneering work by Mukaiyama[5] on the catalytic hydration of alkenes, using Co(acac)2 and oxygen, that sparked wider interest in the field of alkene hydrofunctionalisation. As a result, there now exists an extensive ‘toolkit’ for the addition of hydrogen and a functional group to an alkene with Markovnikov selectivity and high chemo-selectivity using cobalt, manganese and iron complexes.[6,7] Efforts have also been made to extend HAT methodologies to C-C bond formation, both in an intra- and intermolecular fashion: Baran’s group developed a general C-C coupling reaction, utilising electron-deficient alkenes as capable radical acceptors (Scheme 1ai).[8–10] Hydropyridylation of alkenes by intramolecular Minisci reaction was recently demonstrated by Starr,[11] which allows for the formation of structures such as Scheme 1. -

Estimating Fire Properties by Remote Sensing
Estimating Fire Properties by Remote Sensing1. Philip J. Riggan USDA Forest Service Pacific Southwest Research Station 4955 Canyon Crest Drive Riverside, CA 92507 909 680 1534 [email protected] James W. Hoffman Space Instruments, Inc. 4403 Manchester Avenue, Suite 203 Encinitas, CA 92024 760 944 7001 [email protected] James A. Brass NASA Ames Research Center Moffett Federal Airfield, CA 94035 650 604 5232 [email protected] Abstract---Contemporary knowledge of the role of fire in the TABLE OF CONTENTS global environment is limited by inadequate measurements of the extent and impact of individual fires. Observations by 1. INTRODUCTION operational polar-orbiting and geostationary satellites provide an 2. ESTIMATING FIRE PROPERTIES indication of fire occurrence but are ill-suited for estimating the 3. ESTIMATES FROM TWO CHANNELS temperature, area, or radiant emissions of active wildland and 4. MULTI-SPECTRAL FIRE IMAGING agricultural fires. Simulations here of synthetic remote sensing 5. APPLICATIONS FOR FIRE MONITORING pixels comprised of observed high resolution fire data together with ash or vegetation background demonstrate that fire properties including flame temperature, fractional area, and INTRODUCTION radiant-energy flux can best be estimated from concurrent radiance measurements at wavelengths near 1.6, 3.9, and 12 µm, More than 30,000 fire observations were recorded over central Successful observations at night may be made at scales to at Brazil during August 1999 by Advanced Very High Resolution least I km for the cluster of fire data simulated here. During the Radiometers operating aboard polarorbiting satellites of the U.S. daytime, uncertainty in the composition of the background and National Oceanic and Atmospheric Administration. -

Design of SWB Antenna with Triple Band Notch Characteristics for Multipurpose Wireless Applications
applied sciences Article Design of SWB Antenna with Triple Band Notch Characteristics for Multipurpose Wireless Applications Warsha Balani 1, Mrinal Sarvagya 1, Tanweer Ali 2,* , Ajit Samasgikar 3, Saumya Das 4, Pradeep Kumar 5,* and Jaume Anguera 6,7 1 School of Electronics and Communication Engineering, Reva University, Bangalore 560064, India; [email protected] (W.B.); [email protected] (M.S.) 2 Department of Electronics and Communication, Manipal Institute of Technology, Manipal Academy of Higher Education, Manipal, Karnataka 576104, India 3 MMRFIC Technology Pvt Ltd., Bangalore 560016, India; [email protected] 4 Department of Information Technology, Sikkim Manipal Institute of Technology, Sikkim Manipal University, Sikkim 737102, India; [email protected] 5 Discipline of Electrical, Electronic and Computer Engineering, University of KwaZulu-Natal, Durban 4041, South Africa 6 Fractus Antennas, 08174 Barcelona, Spain; [email protected] 7 Electronics and Telecommunication Department, Universitat Ramon LLull, 08022 Barcelona, Spain * Correspondence: [email protected] (T.A.); [email protected] (P.K.) Abstract: A compact concentric structured monopole antenna for super wide band (SWB) applica- tions with triple notch band characteristics is designed and experimentally validated. The antenna covers an immense impedance bandwidth (1.6–47.5 GHz) with sharp triple notch bands at 1.8– 2.2 GHz, 4–7.2 GHz, and 9.8–10.4 GHz to eliminate interference from co-existing advanced wireless services (AWS), C bands, and X bands, respectively. By loading an E-shaped stub connected at the top of the patch and by etching a split elliptical slot at the lower end of the radiating patch, the band rejection characteristics from 1.8–2.2 GHz for the AWS and 4–7.2 GHz for the C band are achieved, Citation: Balani, W.; Sarvagya, M.; respectively.