Harrison's Chapter on Dysphagia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Impact of HIV on Gastroenterology/Hepatology
Core Curriculum: Impact of HIV on Gastroenterology/Hepatology AshutoshAshutosh Barve,Barve, M.D.,M.D., Ph.D.Ph.D. Gastroenterology/HepatologyGastroenterology/Hepatology FellowFellow UniversityUniversityUniversity ofofof LouisvilleLouisville Louisville Case 4848 yearyear oldold manman presentspresents withwith aa historyhistory ofof :: dysphagiadysphagia odynophagiaodynophagia weightweight lossloss EGDEGD waswas donedone toto evaluateevaluate thethe problemproblem University of Louisville Case – EGD Report ExtensivelyExtensively scarredscarred esophagealesophageal mucosamucosa withwith mucosalmucosal bridging.bridging. DistalDistal esophagealesophageal nodulesnodules withwithUniversity superficialsuperficial ulcerationulceration of Louisville Case – Esophageal Nodule Biopsy InflammatoryInflammatory lesionlesion withwith ulceratedulcerated mucosamucosa SpecialSpecial stainsstains forfor fungifungi revealreveal nonnon-- septateseptate branchingbranching hyphaehyphae consistentconsistent withwith MUCORMUCOR University of Louisville Case TheThe patientpatient waswas HIVHIV positivepositive !!!! University of Louisville HAART (Highly Active Anti Retroviral Therapy) HIV/AIDS Before HAART After HAART University of Louisville HIV/AIDS BeforeBefore HAARTHAART AfterAfter HAARTHAART ImmuneImmune dysfunctiondysfunction ImmuneImmune reconstitutionreconstitution OpportunisticOpportunistic InfectionsInfections ManagementManagement ofof chronicchronic ¾ Prevention diseasesdiseases e.g.e.g. HepatitisHepatitis CC ¾ Management CirrhosisCirrhosis NeoplasmsNeoplasms -

General Signs and Symptoms of Abdominal Diseases
General signs and symptoms of abdominal diseases Dr. Förhécz Zsolt Semmelweis University 3rd Department of Internal Medicine Faculty of Medicine, 3rd Year 2018/2019 1st Semester • For descriptive purposes, the abdomen is divided by imaginary lines crossing at the umbilicus, forming the right upper, right lower, left upper, and left lower quadrants. • Another system divides the abdomen into nine sections. Terms for three of them are commonly used: epigastric, umbilical, and hypogastric, or suprapubic Common or Concerning Symptoms • Indigestion or anorexia • Nausea, vomiting, or hematemesis • Abdominal pain • Dysphagia and/or odynophagia • Change in bowel function • Constipation or diarrhea • Jaundice “How is your appetite?” • Anorexia, nausea, vomiting in many gastrointestinal disorders; and – also in pregnancy, – diabetic ketoacidosis, – adrenal insufficiency, – hypercalcemia, – uremia, – liver disease, – emotional states, – adverse drug reactions – Induced but without nausea in anorexia/ bulimia. • Anorexia is a loss or lack of appetite. • Some patients may not actually vomit but raise esophageal or gastric contents in the absence of nausea or retching, called regurgitation. – in esophageal narrowing from stricture or cancer; also with incompetent gastroesophageal sphincter • Ask about any vomitus or regurgitated material and inspect it yourself if possible!!!! – What color is it? – What does the vomitus smell like? – How much has there been? – Ask specifically if it contains any blood and try to determine how much? • Fecal odor – in small bowel obstruction – or gastrocolic fistula • Gastric juice is clear or mucoid. Small amounts of yellowish or greenish bile are common and have no special significance. • Brownish or blackish vomitus with a “coffee- grounds” appearance suggests blood altered by gastric acid. -

Dysphagia Symptoms in People with Diabetes
DYSPHAGIA SYMPTOMS IN PEOPLE WITH DIABETES: A PRELIMINARY REPORT MCKENZIE G. WITZKE Bachelor of Arts in Biology and Psychology The College of Wooster May 2015 submitted in partial fulfillment of requirements for the degree MASTER OF ARTS at the CLEVELAND STATE UNIVERSITY MAY 2020 We hereby approve this thesis For MCKENZIE G. WITZKE Candidate for the Master of Arts degree for the Department of Speech Pathology and Audiology And CLEVELAND STATE UNIVERSITY’S College of Graduate Studies by _______________________________________ Violet Cox Chair, Thesis Committee Department of Speech Pathology and Audiology ________________________________________ Myrita Wilhite Committee member Department of Speech Pathology and Audiology ________________________________________ Anne Su Committee member Department of Health Sciences ___________________April ______________________29, 2020 Date of Defense ACKNOWLEDGEMENTS I wish to express my sincere appreciation to my advisor, Dr. Violet Cox, who has expertly guided me through this process and showed me nothing but patience and support as I navigated this new experience. I would also like to thank Dr. Myrita Wilhite for her encouragement and willingness to provide resources to help me complete this project. Last but not least, I would like to acknowledge the support of my friends and family, who provided consistent camaraderie and encouragement. DYSPHAGIA SYMPTOMS IN PEOPLE WITH DIABETES: A PRELIMINARY REPORT MCKENZIE G. WITZKE ABSTRACT BACKGROUND: Diabetes mellitus is a systemic disease affecting whole-body functioning. The underlying mechanisms and associated concomitant conditions suggest an increased risk for the occurrence of oropharyngeal dysphagia. PURPOSE: This is a qualitative study designed to assess perception of symptoms of oropharyngeal dysphagia in people with diabetes. METHODS: Participants were recruited by word-of-mouth and asked to complete a survey by answering questions on a Likert-type scale indicating the frequency with which they experience each symptom. -

Dysphagia - Pathophysiology of Swallowing Dysfunction, Symptoms, Diagnosis and Treatment
ISSN: 2572-4193 Philipsen. J Otolaryngol Rhinol 2019, 5:063 DOI: 10.23937/2572-4193.1510063 Volume 5 | Issue 3 Journal of Open Access Otolaryngology and Rhinology REVIEW ARTICLE Dysphagia - Pathophysiology of Swallowing Dysfunction, Symptoms, Diagnosis and Treatment * Bahareh Bakhshaie Philipsen Check for updates Department of Otorhinolaryngology-Head and Neck Surgery, Odense University Hospital, Denmark *Corresponding author: Dr. Bahareh Bakhshaie Philipsen, Department of Otorhinolaryngology-Head and Neck Surgery, Odense University Hospital, Sdr. Boulevard 29, 5000 Odense C, Denmark, Tel: +45 31329298, Fax: +45 66192615 the vocal folds adduct to prevent aspiration. The esoph- Abstract ageal phase is completely involuntary and consists of Difficulty swallowing is called dysphagia. There is a wide peristaltic waves [2]. range of potential causes of dysphagia. Because there are many reasons why dysphagia can occur, treatment Dysphagia is classified into the following major depends on the underlying cause. Thorough examination types: is important, and implementation of a treatment strategy should be based on evaluation by a multidisciplinary team. 1. Oropharyngeal dysphagia In this article, we will describe the mechanism of swallowing, the pathophysiology of swallowing dysfunction and different 2. Esophageal dysphagia causes of dysphagia, along with signs and symptoms asso- 3. Complex neuromuscular disorders ciated with dysphagia, diagnosis, and potential treatments. 4. Functional dysphagia Keywords Pathophysiology Dysphagia, Deglutition, Deglutition disorders, FEES, Video- fluoroscopy Swallowing is a complex process and many distur- bances in oropharyngeal and esophageal physiology including neurologic deficits, obstruction, fibrosis, struc- Introduction tural damage or congenital and developmental condi- Dysphagia is derived from the Greek phagein, means tions can result in dysphagia. Breathing difficulties can “to eat” [1]. -

Rational Investigation of Upper Abdominal Pain
THEME UPPER ABDOMINAL PAIN Florian Grimpen Paul Pavli MBBS, Gastroenterology and Hepatology Unit, The PhD, MBBS(Hons), FRACP, Gastroenterology and Canberra Hospital, Australian Capital Territory. Hepatology Unit, The Canberra Hospital, Australian [email protected] Capitol Territory. Rational investigation of upper abdominal pain Upper abdominal pain (UAP) is one of the most common Background presenting symptoms in primary care; the spectrum of possible Upper abdominal pain is a common problem with an causes is wide, and its management can be challenging. extraordinary diversity of possible causes. Many patients have Differential diagnoses range from acute life threatening no structural disease, and making the correct diagnosis can be a challenge. The roles of endoscopy, testing for Helicobacter illnesses such as aortic dissection and myocardial infarction, pylori, and imaging techniques have been debated widely and to relatively benign conditions such as gastro-oesophageal continue to be a matter for discussion. reflux disease (GORD) or functional dyspepsia. Many of the serious conditions are difficult to exclude without elaborate Objective or invasive tests. Unusual causes need to be considered, This article details the value of various investigations in the especially in the young, the immunocompromised, the setting of specific presentations of upper abdominal pain. pregnant, and the elderly. Discussion Functional dyspepsia is a common cause of upper abdominal The prevalence of UAP in western countries is approximately 25% pain but the diagnosis should only be made after consideration when typical reflux symptoms are excluded, or about 40% when of more serious pathology. The various organic causes of included.1,2 The clinical presentation of individual causes of UAP upper abdominal pain and the appropriate investigations are discussed. -

Grading Evidence
Grading Evidence Analysis of the colonoscopic findings in patients with rectal bleeding according to the pattern of their presenting symptoms Journal Diseases of the Colon & Rectum Publisher Springer New York ISSN 0012-3706 (Print) 1530-0358 (Online) Issue Volume 34, Number 5 / May, 1991 Abstract Patients presenting with rectal bleeding were prospectively categorized according to the pattern of their presentation into those with outlet bleeding (n=115), suspicious bleeding (n=59), hemorrhage (n=27), and occult bleeding (n=68). All patients underwent colonoscopy and this was complete in 94 percent. There were 34 patients with carcinoma and 69 with adenomas >1 cm diameter. The percentage of neoplasms proximal to the splenic flexure was 1 percent in outlet bleeding, 24 percent with suspicious bleeding, 75 percent with hemorrhage, and 73 percent with occult bleeding. Barium enema was available in 78 patients and was falsely positive for neoplasms in 21 percent and falsely negative in 45 percent. Colonoscopy is the investigation of choice in patients with suspicious, occult, or severe rectal bleeding. Bleeding of a typical outlet pattern may be investigated by flexible sigmoidoscopy. J Surg Res. 1993 Feb;54(2):136-9. Colonoscopy for intermittent rectal bleeding: impact on patient management. Graham DJ, Pritchard TJ, Bloom AD. Department of Surgery, Case Western Reserve University School of Medicine, Cleveland, Ohio 44106. Abstract Rectal bleeding is a frequent presenting symptom of a number of benign anorectal disorders. However, it may also be a warning sign of more significant gastrointestinal pathology. For this reason, full colonic evaluation has been recommended in patients with intermittent bright red rectal bleeding. -
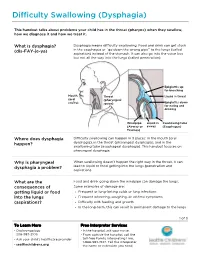
PE3334 Difficulty Swallowing (Dysphagia)
Difficulty Swallowing (Dysphagia) This handout talks about problems your child has in the throat (pharynx) when they swallow, how we diagnose it and how we treat it. What is dysphagia? Dysphagia means difficulty swallowing. Food and drink can get stuck (dis-FAY-je-ya) in the esophagus or “go down the wrong pipe” to the lungs (called aspiration) instead of the stomach. It can also go into the voice box but not all the way into the lungs (called penetration). Epiglottis up for breathing Mouth Throat Liquid in throat (oral (pharyngeal cavity) space) Epiglottis down for eating and drinking Windpipe Liquid in Swallowing tube (Airway or airway (Esophagus) Trachea) Where does dysphagia Difficulty swallowing can happen in 3 places: in the mouth (oral happen? dysphagia), in the throat (pharyngeal dysphagia), and in the swallowing tube (esophageal dysphagia). This handout focuses on pharyngeal dysphagia. Why is pharyngeal When swallowing doesn’t happen the right way in the throat, it can dysphagia a problem? lead to liquid or food getting into the lungs (penetration and aspiration). What are the Food and drink going down the windpipe can damage the lungs. consequences of Some examples of damage are: getting liquid or food • Frequent or long-lasting colds or lung infections into the lungs • Frequent wheezing, coughing, or asthma symptoms (aspiration)? • Difficulty with feeding and growth • In the long-term, this can result in permanent damage to the lungs 1 of 3 To Learn More Free Interpreter Services • Otolaryngology • In the hospital, ask your nurse. 206-987-2105 • From outside the hospital, call the • Ask your child’s healthcare provider toll-free Family Interpreting Line, 1-866-583-1527. -

EGD) Is a Common Endoscopic Procedure Done for Suspected and Proven Lesions of the Upper Gastrointestinal Tract
CLINICAL MEDICAL POLICY Upper Gastrointestinal Endoscopy and Visualization Policy Name: (L34434) Policy Number: MP-073-MC-NC Responsible Department(s): Medical Management Provider Notice Date: 07/01/2018 Issue Date: 08/01/2018 Effective Date: 08/01/2018 Annual Approval Date: 05/16/2019 Revision Date: N/A Products: North Carolina Medicare Assured All participating and nonparticipating hospitals and Application: providers Page Number(s): 1 of 22 DISCLAIMER Gateway Health℠ (Gateway) medical policy is intended to serve only as a general reference resource regarding coverage for the services described. This policy does not constitute medical advice and is not intended to govern or otherwise influence medical decisions. POLICY STATEMENT Gateway Health℠ may provide coverage under the medical-surgical benefits of the Company’s Medicare products for medically necessary Upper Gastrointestinal Endoscopy and Visualization. This policy is designed to address medical necessity guidelines that are appropriate for the majority of individuals with a particular disease, illness or condition. Each person’s unique clinical circumstances warrant individual consideration, based upon review of applicable medical records. Policy No. MP-037-MC-NC Page 1 of 22 PROCEDURES CMS NATIONAL COVERAGE POLICY Title XVIII of the Social Security Act, §1862(a)(1)(A) allows coverage and payment for only those services that are considered to be reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member. Title XVIII of the Social Security Act §1833(e) prohibits Medicare payment for any claim which lacks the necessary information to process the claim. Title XVIII of the Social Security Act §1862(a)(7) excludes routine physical examinations. -

The Gastrointestinal System and the Elderly
2 The Gastrointestinal System and the Elderly Thomas W. Sheehy 2.1. Introduction Gastrointestinal diseases increase with age, and their clinical presenta tions are often confused by functional complaints and by pathophysio logic changes affecting the individual organs and the nervous system of the gastrointestinal tract. Hence, the statement that diseases of the aged are characterized by chronicity, duplicity, and multiplicity is most appro priate in regard to the gastrointestinal tract. Functional bowel distress represents the most common gastrointestinal disorder in the elderly. Indeed, over one-half of all their gastrointestinal complaints are of a functional nature. In view of the many stressful situations confronting elderly patients, such as loss of loved ones, the fears of helplessness, insolvency, ill health, and retirement, it is a marvel that more do not have functional complaints, become depressed, or overcompensate with alcohol. These, of course, make the diagnosis of organic complaints all the more difficult in the geriatric patient. In this chapter, we shall deal primarily with organic diseases afflicting the gastrointestinal tract of the elderly. To do otherwise would require the creation of a sizable textbook. THOMAS W. SHEEHY • Birmingham Veterans Administration Medical Center; and University of Alabama in Birmingham, School of Medicine, Birmingham, Alabama 35233. 63 S. R. Gambert (ed.), Contemporary Geriatric Medicine © Plenum Publishing Corporation 1988 64 THOMAS W. SHEEHY 2.1.1. Pathophysiologic Changes Age leads to general and specific changes in all the organs of the gastrointestinal tract'! Invariably, the teeth show evidence of wear, dis cloration, plaque, and caries. After age 70 years the majority of the elderly are edentulous, and this may lead to nutritional problems. -

Gastroenterology - Outpatient
Gastroenterology - Outpatient Goal Gastroenterology encompasses the evaluation and treatment of patients with disorders of the gastrointestinal tract, pancreas, biliary tract, and liver. It includes disorders of organs within the abdominal cavity and requires knowledge of the manifestations of gastrointestinal disorders in other organ systems, such as the skin. Additional areas include knowledge of nutrition and nutritional deficiencies, and screening and prevention, particularly for colorectal cancer. The general internist should have a wide range of competency in gastroenterology and should be able to provide primary and in some cases secondary preventive care, evaluate a broad array of gastrointestinal symptoms, and manage many gastrointestinal disorders. The general internist is not expected to perform most technical procedures with the important exception of flexible sigmoidoscopy. However, he or she must be familiar with the indications, contraindications, interpretation, and complications of these procedures. Lead Faculty Grace Elta, MD Objectives 1 0 Patient Care and Medical Knowledge 1 1 Dysphagia Differentiate oropharyngeal from esophageal Know the general approach to diagnosis Oropharyngeal dysphagia Use of barium esophagogram/swallowing study Use of endoscopy Use of ENT/speech pathology Know the general approach esophageal dysphagia Use of endoscopy Use of barium esophagogram Know causes of esophageal dysphagia Rings GERD Stricture Pill esophagitis Cancer Know when to include radiology, gastroenterology 1 2 Gastroesophageal -

Oropharyngeal Dysphagia: an Association Between
DOI 10.20398/jscr.v11i1.20955 OROPHARYNGEAL DYSPHAGIA: AN ASSOCIATION BETWEEN DYSPHAGIA LEVEL, SYMPTOMS AND COMORBIDITY DISFAGIA OROFARÍNGEA: ASSOCIAÇÕES ENTRE O GRAU DE DISFAGIA, SINTOMAS E COMORBIDADES Lidiane Maria de Brito Macedo Ferreira¹; Kallil Monteiro Fernandes²; Cynthia Meira de Almeida Godoy³; Hipólito Virgilio Magalhães Junior4; Henrique de Paula Bedaque5. 1. Adjunct Professor at Otorhinolaryngology on Department of Surgery, Federal University of Rio Grande do Norte (UFRN). Natal-RN. Brazil. 2. Otorhinolaryngologist Physician. Natal-RN. Brazil. 3. Speech therapist on EBSERH (Empressa Brasileira de Serviços Hospitalares), UFRN. Natal-RN. Brazil. 4. Adjunct Professor at Department of Speech-Language and Hearing Sciences, UFRN. Natal-RN. Brazil. 5. Physician, Otorhinolaryngology resident. UFRN. Natal-RN. Brazil. Department of Surgery, Federal University of Rio Grande do Norte (UFRN), Brazil. Financial Support: None. Conflict of interest: None. Mailing address: Department of Surgery, Federal University of Rio Grande do Norte (UFRN), AV. Nilo Peçanha 620, Natal – RN, Brazil. E-mail: [email protected]. Submitted: may 18; accepted after revision, may 18, 2020. ABSTRACT Objective: Associate levels of dysphagia according to the patient health condition. Methods: Retrospective study analyzing 149 medical records of patients who underwent Fiberoptic endoscopic evaluation of swallowing (FEES) in a tertiary hospital from 2016 to 2018. Data was collected on symptoms, comorbidities, FESS findings and oropharynx dysphagia classification. Statistical analysis was performed through descriptive and bivariate analysis using the Chi-square and Fisher's exact tests with a 5% significance level. Results: Most patients are elderly, female and with the main complaint of gagging for liquids and solids (30.9%), and gagging only for liquids was associated with the presence of mild dysphagia. -

Geriatric Gi
GERIATRIC GI EDMUNDO RODRIGUEZ- FRIAS, MD “We've put more effort into helping folks reach old age than into helping them enjoy it” GOALS OF TRAINING 1. Pathophysiology of aging. 2. Demographics and epidemiology of aging. 3. Impact of common geriatric disorders on gastroenterology. 4. Social and ethical issues in aging. 5. Listening skills and ethically sound relationship with elderly patient and their families. 6. Communicating bad news to the elderly. 7. Geriatric patients are a very heterogeneous population. 8. Effective strategies for inpatient and outpatient management. 9. Changes in gastrointestinal function with aging. 10. Changes in drug metabolism with aging. 11. Gastrointestinal effects of drugs. (BEERS list) 12. Effect of aging on nutrition. 13. Common gastrointestinal conditions in the elderly. BACKGROUND • Geriatric: >65yo and older, patients of advanced age: >80 years of age. • The U.S. Census Bureau projects the number of Americans >65 yo will more than double between 2010-2050. • Americans >65yo will grow from 13% to more than 20% of the total population by 2030, and the fastest growing segment of this group (individuals > 85) is expected to triple in number over the next four decades. • People living longer and the “baby boomer” generation crossed into the 65 and older age bracket in 2011. • Older adults account for a disproportionate share of healthcare services: o 26% of all physician office visits; o 35% of all hospital stays; o 34% of all prescriptions; o 38% of all emergency medical responses; and o 90% of all nursing home use. • In 2006, elderly underwent 35.3% inpatient and 32.1% outpatient procedures.