UNIVERSITY of ROCHESTER GENETICS DAY Flaum Atrium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spring 2021 Bulletin
Advancing Access to Civil Justice STEPS TOWARD INTERNATIONAL CLIMATE GOVERNANCE Featuring William Nordhaus, Pinelopi Goldberg, and Scott Barrett HONORING WILLIAM LABOV, RUTH LEHMANN , AND GERTRUD SCHÜPBACH SPRING 2021 SELECT UPCOMING VIRTUAL EVENTS May 6 A Conversation with Architect 27 Reflections on a Full, Consequential, Jeanne Gang and Lucky Life: Science, Leadership, Featuring: Jeanne Gang and Education Featuring: Walter E. Massey (left) in conversation with Don Randel (right) June 14 Lessons Learned from Reckoning with Organizational History Featuring: John J. DeGioia, Brent Leggs, Susan Goldberg, Claudia Rankine, and Ben Vinson 13 Finding a Shared Narrative Hosted by the Library of Congress Featuring: Danielle Allen, winner of the Library’s 2020 Kluge Prize Above: “Our Common Purpose” featuring the Juneteenth flag with one star. Artist: Rodrigo Corral For a full and up-to-date listing of upcoming events, please visit amacad.org/events. SPRING 2021 CONTENTS Flooding beside the Russian River on Westside Road in Healdsburg, Sonoma County, California; February 27, 2019. Features 16 Steps Toward International 38 Honoring Ruth Lehmann and Gertrud Climate Governance Schüpbach with the Francis Amory Prize William Nordhaus, Pinelopi Goldberg, and Scott Barrett Ruth Lehmann and Gertrud Schüpbach 30 Honoring William Labov with the Talcott Parsons Prize William Labov CONTENTS 5 Among the contributors to the Dædalus issue on “Immigration, Nativism & Race” (left to right): Douglas S. Massey (guest editor), Christopher Sebastian Parker, and Cecilia Menjívar Our Work 5 Dædalus Explores Immigration, Nativism & Race in the United States 7 Advancing Civil Justice Access in the 21st Century 7 10 New Reports on the Earnings & Job Outcomes of College Graduates 14 Our Common Purpose in Communities Across the Country Members 53 In Memoriam: Louis W. -

Pipsqueak, an Early Acting Member of the Posterior Group of Genes, Affects Vasa Level and Germ Cell-Somatic Cell Interaction in the Developing Egg Chamber
Development 119, 1187-1202 (1993) 1187 Printed in Great Britain © The Company of Biologists Limited 1993 pipsqueak, an early acting member of the posterior group of genes, affects vasa level and germ cell-somatic cell interaction in the developing egg chamber Vivian Siegel*, Thomas A. Jongens, Lily Yeh Jan and Yuh Nung Jan Howard Hughes Medical Institute and Department of Physiology, University of California, San Francisco, CA 94143-0724, USA *Author for correspondence SUMMARY We have identified a new member of the posterior group through vasa; molecular studies indicate that pipsqueak of genes, which we call pipsqueak. We show that affects vasa level in the ovary. We compare vasa and pipsqueak acts after the establishment of the oskar pipsqueak mutant phenotypes in order to determine posterior anchor but before the localization of vasa whether pipsqueak acts solely through vasa, and present protein during oogenesis. Characterization of multiple a model for the role of pipsqueak in posterior pattern alleles at the pipsqueak locus shows that pipsqueak, like formation. vasa, is required for early stages of oogenesis, including but not limited to formation of the egg chamber and pro- gression through Stage 6 of oogenesis. Genetic interac- Key words: Drosophila, oogenesis, posterior pattern formation, tion studies suggest that pipsqueak acts at least partially pipsqueak, vasa INTRODUCTION known collectively as posterior group genes of the grand- childless class, all seem to affect the formation of electron Pattern formation along the anteroposterior axis of the dense particles called polar granules, which are thought to Drosophila melanogaster embryo is initiated prior to fertil- consist of both protein and RNA, and which are localized to ization. -

Phase Transitioned Nuclear Oskar Promotes Cell Division of Drosophila Primordial Germ Cells
bioRxiv preprint doi: https://doi.org/10.1101/397992; this version posted August 22, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. PHASE TRANSITIONED NUCLEAR OSKAR PROMOTES CELL DIVISION OF DROSOPHILA PRIMORDIAL GERM CELLS Kathryn E. Kistler*1,2, Tatjana Trcek*†1, Thomas R. Hurd1, Ruoyu Chen1, Feng-Xia Liang3, Joseph Sall3, Masato Kato4, Ruth Lehmann†1 1: HHMI, Skirball Institute of Biomolecular Medicine, Department of Cell Biology, NYU School of Medicine, NY, USA 2: Department of Molecular and Cellular Biology, University of Washington, WA, USA 3: DART Microscopy Laboratory, NYU Langone Health, NY, USA 4: Department of Biochemistry, University of Texas Southwestern Medical Center, TX, USA ORCID ID Trcek Tatjana: 0000-0003-4405-8733 *: equal contribution †: correspondence ([email protected]; [email protected]) ABSTRACT Germ granules are non-membranous ribonucleoprotein granules deemed the hubs for post-transcriptional gene regulation and functionally linked to germ cell fate across species. Little is known about the physical properties of germ granules and how these relate to germ cell function. Here we study two types of germ granules in the Drosophila embryo: cytoplasmic germ granules that instruct primordial germ cells (PGCs) formation and nuclear germ granules within early PGCs with unknown function. We show that cytoplasmic and nuclear germ granules are phase transitioned condensates nucleated by Oskar protein that display liquid as well as hydrogel-like properties. -

Annual Report Fy 2018 Human Frontier Science Program Organization
APRIL 2017 APRIL 2018 — MARCH 2019 ANNUAL REPORT FY 2018 HUMAN FRONTIER SCIENCE PROGRAM ORGANIZATION The Human Frontier Science Program Organization (HFSPO) is unique, supporting international collaboration to undertake innovative, risky, basic research at the frontier of the life sciences. Special emphasis is given to the support and training of independent young investigators, beginning at the postdoctoral level. The Program is implemented by an international organisation, supported financially by Australia, Canada, France, Germany, India, Italy, Japan, the Republic of Korea, New Zealand, Norway, Singapore, Switzerland, the United Kingdom of Great Britain and Nothern Ireland, the United States of America, and the European Commission. Since 1990, over 7000 researchers from more than 70 countries have been supported. Of these, 28 HFSP awardees have gone on to receive the Nobel Prize. 2 The following documents are available on the HFSP website www.hfsp.org: Joint Communiqués (Tokyo 1992, Washington 1997, Berlin 2002, Bern 2004, Ottawa 2007, Canberra 2010, Brussels 2013, London 2016): https://www.hfsp.org/about/governance/membership Statutes of the International Human Frontier Science Program Organization: https://www.hfsp.org/about/governance/hfspo-statutes Guidelines for the participation of new members in HFSPO: https://www.hfsp.org/about/governance/membership General reviews of the HFSP (1996, 2001, 2006-2007, 2010, 2018): https://www.hfsp.org/about/strategy/reviews Updated and previous lists of awards, including titles and abstracts: -

October 11, 1994, NIH Record, Vol. XLVI, No. 21
October l 1, 1994 Vol. XLVI No. 21 "Still U.S. Department of Health The Second and Human Services Best Thing About Payday" National Institutes of Health Dunbar To Give First Pittman Lecture, Oct. 26 By Sara Byars scientist recognized internarionally for A her pioneering work in contraceptive vaccines has been selected to deliver the first Margaret Pittman Lecture, a new NIH series chat honors outstanding women scientists. Dr. Bonnie S. Dunbar, professor of cell . :/ biology and obstetrics and gynecology at Baylor College of Medicine, will speak on "New ':'' .:' f ,; ... .. Fromiers in Reproductive Biology and I !- J ~ , • I , , ' f ., ' / , , Contraceptive Vaccines" at 3 p.m. on 0cc. 26 ' • /} : 4 t ., : in Masur Auditorium, Bldg. I 0 . ' ' . ! • ' I I I • Guiding che development of che Pittman • ' ' ,• ,: k'I I ' I lectureship series is the NIH women scientists J: • ! • •'I I It♦ l'- : ,4 • • •· ... / ;~ ~ advisory committee, a group char advises At the NIH Research Festival 1994 poster session, Dr. Lynn Hudson (r), chief. molecular genetics section scientific directors on matters pertaining co rhe ofNJNDS' Laboratory ofViral and Molecular Pathogenesis, stops by to view the work ofDr. Rosemary role of women scientists at NIH. Wong, one ofthe authors who works in NIDDK's Molemlar, Cellular and Endocrinology Branch. See This lectureship honors Or. Margaret additional coverage ofthe event on Pages 6-7. Pittman, rhe first woman co hold the position (See PITTMAN LECTURE, Page 2) NIGMS Reorganizes, Moves to Natcher Bldg. is month, che N acional Institute of General Medical Sciences is undergoing a reorganiza Vitetta Is NIAID's 1994 tion and a move to che new William H. -

Friday, September 19
Building the New York Stem Cell Community CUNY Graduate Center PROGRAM COMMITTEE Ira S. Cohen, M.D., Ph.D., Stony Brook University Steven Goldman, M.D., Ph.D., University of Rochester Ruth Lehmann, Ph.D., New York University School of Medicine Ihor Lemischka, Ph.D., Mount Sinai School of Medicine Lorenz Studer, M.D., Sloan-Kettering Institute Gordana Vunjak-Novakovic, Ph.D., Columbia University Scott Noggle, Ph.D., New York Stem Cell Foundation (Facilities Workshop) Sally Temple, Ph.D., New York Neural Stem Cell Institute (Facilities Workshop) ORGANIZING COMMITTEE David Anders, Ph.D., Chair Chief Scientific Officer, NYSTEM Linda Tripoli, R.N., Program Coordinator, NYSTEM Matthew Kohn, Ph.D., Scientific Officer, NYSTEM Kathy Chou, Ph.D., Scientific Officer, NYSTEM Marti McHugh, Director, Clinical Program Development Virginia Reyes The Wadsworth Center Photography and Illustrations Unit Tracy Godfrey PROGRAM-AT-A-GLANCE PROGRAM-AT-A-GLANCE MAY 26 – Elebash Recital Hall 8:00 AM Registration Opens and Continental Breakfast – Recital Hall Lobby 9:00 AM Shared Equipment and Facilities Workshop – Part 1 10:15 AM Break 10:30 AM Shared Equipment and Facilities Workshop – Part 2 12:00 PM Lunch – Concourse Lobby (one floor down) 1:20 PM Opening Remarks 1:30 PM Session I: Neural Stem Cells 3:00 PM Break 3:30 PM Session II: Stem Cells and Cancer 5:00 PM Reception/Poster Session – Concourse Lobby (one floor down) MAY 27 – Elebash Recital Hall 8:00 AM Registration Opens and Continental Breakfast – Recital Hall Lobby 9:00 AM Session III: Stem Cells in Diseases 10:30 AM Break 11:00 AM Session IV: Stem Cell Biology 12:30 PM Lunch – Ninth Floor Conference Room 1:30 PM Session V: Tissue Engineering and Technology 3:00 PM Break 3:30 PM Session VI: Pluripotency and Reprogramming 5:00 PM Adjourn WELCOME STATE OF NEW YORK DEPARTMENT OF HEALTH Wadsworth Center The Governor Nelson A. -

About Whitehead Institute for Biomedical Research Selected
About Whitehead Institute for Biomedical Research Selected Achievements in FOUNDING VISION Biomedical Science Whitehead Institute is a nonprofit, independent biomedical research institute with pioneering programs in cancer research, developmental biology, genetics, and Isolated the first tumor suppressor genomics. It was founded in 1982 through the generosity of Edwin C. "Jack" Whitehead, gene, the retinoblastoma gene, and a businessman and philanthropist who sought to create a new type of research created the first genetically defined institution, one that would exist outside the boundaries of a traditional academic human cancer cells. (Weinberg) institution, and yet, through a teaching affiliation with the Massachusetts Institute of Technology (MIT), offer all the intellectual, collegial, and scientific benefits of a leading Isolated key genes involved in diabetes, research university. hypertension, leukemia, and obesity. (Lodish) WHITEHEAD INSTITUTE TODAY True to its founding vision, the Institute gives outstanding investigators broad freedom Mapped and cloned the male- to pursue new ideas, encourages novel collaborations among investigators, and determining Y chromosome, revealing a accelerates the path of scientific discovery. Research at Whitehead Institute is unique self-repair mechanism. (Page) conducted by 22 principal investigators (Members and Fellows) and approximately 300 visiting scientists, postdoctoral fellows, graduate students, and undergraduate Developed a method for genetically students from around the world. Whitehead Institute is affiliated with MIT in its engineering salt- and drought-tolerant teaching activities but wholly responsible for its own research programs, governance, plants. (Fink) and finance. Developed the first comprehensive cellular LEADERSHIP network describing how the yeast Whitehead Institute is guided by a distinguished Board of Directors, chaired by Sarah genome produces life. -

Mage, Rose G. 2018 Dr
Mage, Rose G. 2018 Dr. Rose G. Mage Oral History Download the PDF: Mage_Rose_oral_history (130 kB) Office of NIH History and Stetten Museum Oral History with Dr. Rose Mage, NIAID August 22, 2018 GM: I am Dr. Gordon Margolin, volunteer in the Office of NIH History and Stetten Museum about to do an oral history with Dr. Rose G. Mage. She served as a Career Investigator in the NIAID Lab of Immunology, starting in 1965, and then as the Section Chief of the Molecular Immunogenetics section from 1988 until her retirement in 2008. We are here in the NIH Library’s audio-visual recording center on August 22, 2018. Thank you, Dr. Mage, for agreeing to record this history about you, your scientific accomplishments, and the time you spent here at NIH. Let’s begin by asking you to tell me a bit of your background, where you were raised, something about your family, and your educational pathway. RGM: I was born and raised in New York City. My father was an immigrant from Romania. He arrived in New York when he was 5 years old in 1905. I was named after his mother who died from typhoid in the 1920s. My mother was born in the USA but her family, three of her four older brothers were immigrants. I decided I wanted to be a scientist when I was about 9 years old, did well in public schools and skipped grades twice. I applied to the Bronx High School of Science and was accepted after taking a written test for admission. -

Timeline of Immunology
TIMELINE OF IMMUNOLOGY 1549 – The earliest account of inoculation of smallpox (variolation) occurs in Wan Quan's (1499–1582) 1718 – Smallpox inoculation in Ottoman Empire realized by West. Lady Mary Wortley Montagu, the wife of the British ambassador to Constantinople, observed the positive effects of variolation on the native population and had the technique performed on her own children. 1796 – First demonstration of smallpox vaccination (Edward Jenner) 1837 – Description of the role of microbes in putrefaction and fermentation (Theodore Schwann) 1838 – Confirmation of the role of yeast in fermentation of sugar to alcohol (Charles Cagniard-Latour) 1840 – Proposal of the germ theory of disease (Jakob Henle) 1850 – Demonstration of the contagious nature of puerperal fever (childbed fever) (Ignaz Semmelweis) 1857–1870 – Confirmation of the role of microbes in fermentation (Louis Pasteur) 1862 – Phagocytosis (Ernst Haeckel) 1867 – Aseptic practice in surgery using carbolic acid (Joseph Lister) 1876 – Demonstration that microbes can cause disease-anthrax (Robert Koch) 1877 – Mast cells (Paul Ehrlich) 1878 – Confirmation and popularization of the germ theory of disease (Louis Pasteur) 1880 – 1881 -Theory that bacterial virulence could be attenuated by culture in vitro and used as vaccines. Proposed that live attenuated microbes produced immunity by depleting host of vital trace nutrients. Used to make chicken cholera and anthrax "vaccines" (Louis Pasteur) 1883 – 1905 – Cellular theory of immunity via phagocytosis by macrophages and microphages (polymorhonuclear leukocytes) (Elie Metchnikoff) 1885 – Introduction of concept of a "therapeutic vaccination". Report of a live "attenuated" vaccine for rabies (Louis Pasteur and Pierre Paul Émile Roux). 1888 – Identification of bacterial toxins (diphtheria bacillus) (Pierre Roux and Alexandre Yersin) 1888 – Bactericidal action of blood (George Nuttall) 1890 – Demonstration of antibody activity against diphtheria and tetanus toxins. -

Schedule of C Ourses
2020–2021 Schedule of Courses Schedule The David Rockefeller Graduate Program offers a multiple sclerosis); perception, cognition, and memory (autism, schizophrenia, and Alzheimer’s disease); consciousness (coma selection of courses, many of which students can and persistent vegetative state); mood (depression and anxiety); choose based on their interests and area of thesis motivation (addiction); sensation (pain); motor control (Parkinson’s research. Organized by Rockefeller faculty, and taught disease and ataxia); and trauma (brain or spinal cord injury and stroke). by scientists at the top of their fields, both from within Class length and frequency: Two-hour session, once weekly and outside of the university, these courses provide a Method of evaluation: Attendance, participation in the discussions, stimulating and dynamic curriculum that students can student presentations, and a final speculative paper relating a tailor to fit their personal goals, in consultation with disordered trait to a specific brain circuit the dean of graduate studies. Cell Biology SANFORD M. SIMON and SHAI SHAHAM Biochemical and Biophysical Methods, I & II This advanced course covering major topics in modern cell biology is GREGORY M. ALUSHIN, SETH A. DARST, SHIXIN LIU, and MICHAEL P. ROUT taught by faculty and visitors who are specialists in various disciplines. This course presents the fundamental principles of biochemistry Class length and frequency: Three-hour lecture, once weekly; and biophysics, with an emphasis on methodologies. In addition, two-hour discussion, twice weekly case studies are discussed, examining how physical and chemical methods have been used to establish the molecular mechanisms Prerequisite(s): Good knowledge of textbook cell biology of fundamental biological processes. -
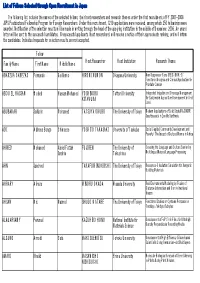
List of Fellows Selected Through Open Recruitment in Japan
List of Fellows Selected through Open Recruitment in Japan The following list includes the names of the selected fellows, their host researchers and research themes under the first recruitment of FY 2007-2008 JSPS Postdoctoral Fellowship Program for Foreign Researchers. Under this recruitment, 1219 applications were received, among which 250 fellowships were awarded. Notification of the selection results will be made in writing through the head of the applying institution in the middle of December, 2006. An award letter will be sent to the successful candidates. Unsuccessful applicants (host researchers) will receive a notice of their approximate ranking, and will inform the candidates. Individual requests for selection results are not accepted. Fellow Family Name First Name Middle Name Host Researcher Host Institution Research Theme ABARZUA CABEZAS Fernando Guillermo HIROMI KUMON Okayama University New Suppressor Gene (REIC/DKK-3): Functional Analysis and Clinical Application for Prostate Cancer ABOU EL HASSAN Waleed Hassan Mohamed YOSHINOBU Tottori University Integrated Irrigation and Drainage Management KITAMURA for Sustainable Agriculture Development in Arid Land ABUBAKAR Saifudin Mohamed TATSUYA OKUBO The University of Tokyo Modern Applications of Solid State MAS NMR Spectroscopy in Zeolite Synthesis ADI Alpheus Bongo Chimaeze YOSHITO TAKASAKI University of Tsukuba Social Capital, Community Development and Poverty: The Impact of Cultural Norms in Africa AHMED Mohamed Abdel Fattah FUJI REN The University of Crossing the Language and Culture Barrier by Ibrahim Tokushima Multilingual Natural Language Processing AHN Jaecheol TAKAFUMI NOGUCHI The University of Tokyo Resources-Circulation Simulation for Inorganic Building Materials AHRARY Alireza MINORU OKADA Waseda University Real Environments Modeling by Fusion of Distance Information and Omni-directional Images AHSAN Md. -

Phase Separated Assemblies in Cell Biology
MEETING AGENDA Phase Separated Assemblies in Cell Biology The Banbury Center, Cold Spring Harbor Laboratory, New York, USA December 16-19, 2018 This meeting was funded by the Cold Spring Harbor Laboratory Corporate Sponsor Program Organizers: Arup K. Chakraborty, Massachusetts Institute of Technology; Geraldine Seydoux, Johns Hopkins University; Richard Young, Whitehead Institute for Biomedical Research; with Consulting Organizer, Phillip Sharp, Koch Institute for Integrative Cancer Research This Banbury meeting brought together an interdisciplinary group of experts to: review functions and types of phase-separated assemblies in biology; develop a common conceptual framework and nomenclature; identify molecular code characteristics underlying assemblies; consider pathologies caused by aberrant phase-separated assemblies; and examine manipulation of phase- separated assemblies as a novel treatment target. SUNDAY, DECEMBER 16 Afternoon Participant arrivals 6:00 pm Reception, dinner MONDAY, DECEMBER 17 7:15 am Breakfast at Robertson House 8:30 am Welcoming and Introductory Remarks Rebecca Leshan, The Banbury Center, Cold Spring Harbor Laboratory Arup Chakraborty, Massachusetts Institute of Technology, Cambridge, USA Geraldine Seydoux, Johns Hopkins University, Baltimore, USA Rick Young, Whitehead Institute for Biomedical Research, Cambridge, USA December 16-19, 2018 | Phase Separated Assemblies in Cell Biology | 1 8:45 am SESSION 1: Physical Principles Clifford Brangwynne, Princeton University, Princeton, USA Arup Chakraborty, Massachusetts