Synthesis and Development of Long-Acting Abacavir Prodrug Nanoformulations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download Article PDF/Slides
Kan Lu, PharmD New Antiretrovirals for Based on a presentation at prn by Roy M. Gulick, md, mph the Treatment of HIV: Kan Lu, PharmD | Drug Development Fellow University of North Carolina School of Pharmacy Chapel Hill, North Carolina The View in 2006 Roy M. Gulick, md, mph Reprinted from The prn Notebook® | october 2006 | Dr. James F. Braun, Editor-in-Chief Director, Cornell Clinical Trials Unit | Associate Professor of Medicine, Meri D. Pozo, PhD, Managing Editor. Published in New York City by the Physicians’ Research Network, Inc.® Weill Medical College of Cornell University | New York, New York John Graham Brown, Executive Director. For further information and other articles available online, visit http://www.prn.org | All rights reserved. ©october 2006 substantial progress continues to be made in the arena of cokinetics and a long extracellular half-life of approximately 10 hours antiretroviral drug development. prn is again proud to present its annual (Zhu, 2003). During apricitabine’s development, a serious drug interac- review of the experimental agents to watch for in the coming months and tion with lamivudine (Epivir) was noted. Although the plasma years. This year’s review is based on a lecture by Dr. Roy M. Gulick, a long- concentrations of apricitabine were unaffected by coadministration of time friend of prn, and no stranger to the antiretroviral development lamivudine, the intracellular concentrations of apricitabine were reduced pipeline. by approximately sixfold. Additionally, the 50% inhibitory concentration To date, twenty-two antiretrovirals have been approved by the Food (ic50) of apricitabine against hiv with the M184V mutation was increased and Drug Administration (fda) for the treatment of hiv infection. -

Ep 2531027 B1
(19) TZZ ¥_Z _T (11) EP 2 531 027 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 31/4985 (2006.01) A61K 31/52 (2006.01) 06.05.2015 Bulletin 2015/19 A61K 31/536 (2006.01) A61K 31/513 (2006.01) A61K 38/55 (2006.01) A61P 31/18 (2006.01) (21) Application number: 11737484.3 (86) International application number: (22) Date of filing: 24.01.2011 PCT/US2011/022219 (87) International publication number: WO 2011/094150 (04.08.2011 Gazette 2011/31) (54) Therapeutic combination comprising dolutegravir, abacavir and lamivudine Therapeutische Zusammensetzung enthaltend Dolutegravir, Abacavir und Lamivudine Combinaison thérapeutique comprenant du dolutégravir, de l’abacavir et de la lamivudine (84) Designated Contracting States: (74) Representative: Gladwin, Amanda Rachel AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GlaxoSmithKline GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Global Patents (CN925.1) PL PT RO RS SE SI SK SM TR 980 Great West Road Designated Extension States: Brentford, Middlesex TW8 9GS (GB) BA ME (56) References cited: (30) Priority: 27.01.2010 US 298589 P WO-A1-2010/011812 WO-A2-2009/148600 US-A1- 2006 084 627 US-A1- 2006 084 627 (43) Date of publication of application: US-A1- 2008 076 738 US-A1- 2009 318 421 12.12.2012 Bulletin 2012/50 US-A1- 2009 318 421 US-B1- 6 544 961 (73) Proprietor: VIIV Healthcare Company • SONG1 et al: "The Effect of Ritonavir-Boosted Research Triangle Park, NC 27709 (US) ProteaseInhibitors on the HIV Integrase Inhibitor, S/GSK1349572,in Healthy Subjects", INTERNET , (72) Inventor: UNDERWOOD, Mark, Richard 15 September 2009 (2009-09-15), XP002697436, Research Triangle Park Retrieved from the Internet: URL:http: North Carolina 27709 (US) //www.natap.org/2009/ICCAC/ICCAC_ 52.htm [retrieved on 2013-05-21] Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -

Trends in Antiretroviral Treatment in Australia
AUSTRALIAN HIV OBSERVATIONAL DATABASE (AHOD) ANNUAL REPORT (Volume 20, Number 1: December 2020) 2020 Clinical characteristics of overseas-born men who have sex with men (MSM) in the AHOD cohort and implications for clinical practice In Australia HIV notifications are increasing among overseas-born men who have sex with men (MSM), particularly among Asian-born MSM. Australian evidence suggests that culturally and/or linguistically diverse populations are less likely to start treatment early irrespective of CD4 cell count at diagnosis, but little is known about response once in care. Using data from AHOD, Jolie L Hutchinson and colleagues (2020) compared treatment response in overseas-born MSM from non-English-speaking countries with Australian-born MSM, further categorised based on participation in the Australian Temporary Residents Access Study (ATRAS) which provide temporary residents ineligible for Medicare, access to HIV treatment. ATRAS patients were chosen as the closest surrogate to identifying newly arrived overseas-born MSM. The authors explored the time to first virological suppression (VS) (viral load (VL) <400 copies/mL) and time to virological failure (VF) (>400 copies/mL after suppression). CD4 cell counts and VL measurements were taken at treatment initiation. Adjusted Hazard Ratios (HR) are reported with 95% CI. Results, as shown in figure 1, indicate that overseas-born MSM did not differ significantly in the rate of VS or in the rate of first VF after suppression. This result is different from findings in other settings, and differences may, in part, be explained by the nature of healthcare provision. In Australia, all residents can access ART for free or with a small co-payment; those ineligible for Medicare can get pharmaceutical company-provided ART which is not necessarily straightforward for non-English speakers. -
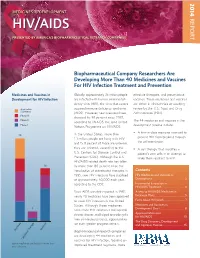
14-258 Phrma HIV/AIDS2014 0819.Indd
2014 MEDICINES IN DEVELOPMENT REPORT HIV/AIDS PRESENTED BY AMERICA’S BIOPHARMACEUTICAL RESEARCH COMPANIES Biopharmaceutical Company Researchers Are Developing More Than 40 Medicines and Vaccines For HIV Infection Treatment and Prevention Medicines and Vaccines in Globally, approximately 35 million people effective therapies, and preventative Development for HIV Infection are infected with human immunodefi - vaccines. These medicines and vaccines ciency virus (HIV), the virus that causes are either in clinical trials or awaiting Application acquired immune defi ciency syndrome review by the U.S. Food and Drug Submitted (AIDS). However, new infections have Administration (FDA). Phase III dropped by 38 percent since 2001, Phase II The 44 medicines and vaccines in the according to UNAIDS, the Joint United Phase I development pipeline include: Nations Programme on HIV/AIDS. • A fi rst-in-class medicine intended to In the United States, more than 25 prevent HIV from breaking through 1.1 million people are living with HIV the cell membrane. and 15.8 percent of those are unaware they are infected, according to the • A cell therapy that modifi es a U.S. Centers for Disease Control and patient’s own cells in an attempt to Prevention (CDC). Although the U.S. make them resistant to HIV. HIV/AIDS-related death rate has fallen 16 by more than 80 percent since the introduction of antiretroviral therapies in Contents 1995, new HIV infections have stabilized HIV Medicines and Vaccines in at approximately 50,000 each year, Development ......................................2 according to the CDC. Incremental Innovation in HIV/AIDS Treatment .......................... 4 Since AIDS was fi rst reported in 1981, Access to HIV/AIDS Medicines in nearly 40 medicines have been approved Exchange Plans ...................................5 to treat HIV infection in the United Facts About HIV/AIDS ........................7 States. -

Current Status and Prospects of HIV Treatment
Available online at www.sciencedirect.com ScienceDirect Current status and prospects of HIV treatment 1 2 Tomas Cihlar and Marshall Fordyce Current antiviral treatments can reduce HIV-associated at suppressing plasma viremia, and significant progress morbidity, prolong survival, and prevent HIV transmission. has been made toward regimen simplification through the Combination antiretroviral therapy (cART) containing preferably combination of three active drugs into single-tablet regi- three active drugs from two or more classes is required for mens (STRs) (Figure 1) and optimization of drug profiles durable virologic suppression. Regimen selection is based on that maximize long-term tolerability and safety. Lifelong, virologic efficacy, potential for adverse effects, pill burden and chronic therapy without treatment interruption is the dosing frequency, drug–drug interaction potential, resistance standard of care, and the availability of multiple effective test results, comorbid conditions, social status, and cost. With drugs in several classes with differing resistance, safety, prolonged virologic suppression, improved clinical outcomes, and tolerability profiles provides choices after failure of and longer survival, patients will be exposed to antiretroviral first-line treatment. agents for decades. Therefore, maximizing the safety and tolerability of cART is a high priority. Emergence of resistance Herein, we review the current status of antiretroviral and/or lack of tolerability in individual patients require therapy and guidelines for HIV-infected adults, as well availability of a range of treatment options. Development of new as prospects for further innovation in HIV treatment to drugs is focused on improving safety (e.g. tenofovir benefit patients and optimize their long-term health. alafenamide) and/or resistance profile (e.g. -

Wibke E. Diederich Holger Steuber Editors Therapy of Viral Infections 15 Topics in Medicinal Chemistry
Topics in Medicinal Chemistry 15 Wibke E. Diederich Holger Steuber Editors Therapy of Viral Infections 15 Topics in Medicinal Chemistry Editorial Board: P. R. Bernstein, Rose Valley, USA A. Buschauer, Regensburg, Germany G. I. Georg, Minneapolis, USA J. A. Lowe, Stonington, USA U. Stilz, Malov, Denmark Prof. Dr. C. T. Supuran, Sesto Fiorentino (Firenze), Italy A. K. Saxena, Lucknow, India Aims and Scope Drug research requires interdisciplinary team-work at the interface between chemistry, biology and medicine. Therefore, the new topic-related series Topics in Medicinal Chemistry will cover all relevant aspects of drug research, e.g. pathobiochemistry of diseases, identification and validation of (emerging) drug targets, structural biology, drugability of targets, drug design approaches, chemogenomics, synthetic chemistry including combinatorial methods, bioorganic chemistry, natural compounds, high-throughput screening, pharmacological in vitro and in vivo investigations, drug-receptor interactions on the molecular level, structure-activity relationships, drug absorption, distribution, metabolism, elimina- tion, toxicology and pharmacogenomics. In general, special volumes are edited by well known guest editors. In references Topics in Medicinal Chemistry is abbreviated Top Med Chem and is cited as a journal. More information about this series at http://www.springer.com/series/7355 Wibke E. Diederich • Holger Steuber Editors Therapy of Viral Infections With contributions by E. Bo¨ttcher-Friebertsha¨user Á U. Chiacchio Á F. Christ Á E. Crespan Á J. Demeulemeester Á Z. Debyser Á U. Dietrich Á W.E. Diederich Á R. Du¨rr Á F.G.R. Ehlert Á C. Frezza Á A. Garbelli Á W. Garten Á S.V. Giofre` Á K. Hardes Á M. -

Background Paper 6.7 Human Immunodeficiency Virus (HIV)/ Acquired Immune Deficiency Syndromes (AIDS)
Priority Medicines for Europe and the World "A Public Health Approach to Innovation" Update on 2004 Background Paper Written by Warren Kaplan Background Paper 6.7 Human Immunodeficiency Virus (HIV)/ Acquired Immune Deficiency Syndromes (AIDS) By Warren Kaplan, Ph.D., JD, MPH 15 February 2013 Update on 2004 Background Paper, BP 6.7 HIV/AIDS Table of Contents What is new since 2004? ..................................................................................................................................... 4 1. Introduction ................................................................................................................................................. 7 2. What are the Epidemiological Trends for Europe and the World? ................................................... 7 2.1 Western and Central Europe ............................................................................................................. 7 2.2 Eastern Europe .................................................................................................................................... 9 2.2 The World (including Europe) ........................................................................................................ 11 3. What is the Control Strategy? Is There an Effective Package of Control Methods Assembled into a “Control Strategy” for Most Epidemiological Settings?................................................................. 13 3.1 Is there a pharmaceutical ‘gap’? .................................................................................................... -

Can the Further Clinical Development of Bevirimat Be Justified?
SwatiCE: Swati; QAD/201969; Total nos of Pages: 2; QAD 201969 EDITORIAL COMMENT Can the further clinical development of bevirimat be justified? Mark A. Wainberga and Jan Albertb AIDS 2010, 24:000–000 Keywords: bevirimat, protease inhibitor resistance HIV-infected patients who fail standard therapeutic Not surprisingly, however, HIV resistance against BVM regimens are in need of new drugs that will remain has been selected in tissue culture. Mutations responsible active against drug-resistant viral variants. There is a for resistance have been identified within flanking constant need to develop new compounds that will not be sequences of the p24/p2 cleavage site and have been compromised by problems of cross-resistance, as so often confirmed as possessing biological relevance by site- occurs among members of the same family of drugs, for directed mutagenesis [3]. These mutations can sometimes example, nucleoside reverse transcriptase inhibitors interfere with the ability of BVM to bind to its target, (NRTIs), nonnucleoside reverse transcriptase inhibitors although, in most cases, increased cleavage efficiency at (NNRTIs), and protease inhibitors. Indeed, successful the p24/p2 junction may also occur [3,4]. second-line and salvage regimens will commonly include members of drug classes to which a patient has not Recent results have revealed that naturally occurring previously been exposed, for example, a protease inhibi- polymorphisms located within p2, close to the p24 tor and an integrase strand transfer inhibitor (INSTI) in cleavage site, may be present in some individuals, the case of an individual who began therapy with two resulting in lower BVM anti-HIV efficacy. In addition, NRTIs and a NNRTI, as well as the use of fusion clinical investigators have long suspected that BVM might inhibitors, for example, enfuvirtide, and CCR5 antago- potentially be less effective in the treatment of patients nists, for example, maraviroc. -

Structural Aspects of Drug Resistance and Inhibition of HIV-1 Reverse Transcriptase
Viruses 2010, 2, 606-638; doi:10.3390/v2020606 OPEN ACCESS viruses ISSN 1999-4915 www.mdpi.com/journal/viruses Review Structural Aspects of Drug Resistance and Inhibition of HIV-1 Reverse Transcriptase Kamalendra Singh, Bruno Marchand, Karen A. Kirby, Eleftherios Michailidis and Stefan G. Sarafianos * Christopher Bond Life Sciences Center & Department of Molecular Microbiology & Immunology, University of Missouri, Columbia, Missouri 65211, USA; E-Mails: [email protected] (K.S.); [email protected] (B.M.); [email protected] (K.A.K.); [email protected] (E.M.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-573-882-4338. Received: 2 December 2009; in revised form: 22 January 2010 / Accepted: 3 February 2010 / Published: 11 February 2010 Abstract: HIV-1 Reverse Transcriptase (HIV-1 RT) has been the target of numerous approved anti-AIDS drugs that are key components of Highly Active Anti-Retroviral Therapies (HAART). It remains the target of extensive structural studies that continue unabated for almost twenty years. The crystal structures of wild-type or drug-resistant mutant HIV RTs in the unliganded form or in complex with substrates and/or drugs have offered valuable glimpses into the enzyme’s folding and its interactions with DNA and dNTP substrates, as well as with nucleos(t)ide reverse transcriptase inhibitor (NRTI) and non-nucleoside reverse transcriptase inhibitor (NNRTIs) drugs. These studies have been used to interpret a large body of biochemical results and have paved the way for innovative biochemical experiments designed to elucidate the mechanisms of catalysis and drug inhibition of polymerase and RNase H functions of RT. -

NRTI Toxicity Francois Venter Wits Reproductive Health & HIV Institute
NRTI toxicity Francois Venter Wits Reproductive Health & HIV Institute Pipeline Report http://www.pipelinereport.org So what we got? Current and Investigational Antiretrovirals NRTIs/NtRTI NNRTI PI Integrase In Abacavir Doravirine Atazanavir Cabotegravir Didanosine Efavirenz Darunavir Dolutegravir Emtricitabine Etravirine Fosamprenavir Elvitegravir Lamivudine Nevirapine Indinavir Raltegravirr Stavudine Rilpivirine Lopinavir/r Tenofovir Fixed Combo Nelfinavir Tenofovir AF MI AZT/3TC Ritonavir Zidovudine Saquinavir ABC/3TC Bevirimat Tipranavir TDF/FTC BMS-955176 CYP3A4 In AZT/3TC/ABC EI/FI Cobicistat TDF/FTC/EFV Enfuvirtide TDF/FTC/RPV Maraviroc TDF/FTC/ETG/COB BMS-663068 Thanks Howard Kessler ABC/3TC/DTG TAF/FTC/ETG/COB • 28 approved drugs, 5 classes • Up to 10 recommended first-line regimens • BUT: only 4 NRTIs that matter (if leave out 3TC/FTC) An aside… • Nuke free/sparing initial ART NNRTI 1 PI + 1 NNRTI Efavirenz INSTI PI 1 PI + 1 INSTI Raltegravir Atazanavir/r Darunavir/r Lopinavir/r NRTI 1 PI + 1 NRTI Lamivudine FI 1 PI + 1 FI Maraviroc Nuke free/sparing initial ART Study Regimen Comparison Efficacy SPARTAN ATV/r + RAL ATV/r + TDF-FTC HIV-RNA <50 at wk 24: 74.6% vs 63.3% non-inferior (but high bilirubinemia and resistance to RAL) A4001078 ATV/r + MVC ATV/r + TDF-FTC HIV-RNA <50 at wk 48: 74.6% vs 83.6% non-inferior NEAT001/ DRV/r + RAL DRV/r + TDF-FTC Virological or clinical failure at wk 96: ANRS143 17.8% vs 13.8% non-inferior significantly inferior to standard therapy if CD4 <200/ml; trend for >100 000 c/mL MODERN DRV/r + MVC -

2013 Prioritisation Report Priority Antiretrovirals for the Medicines
PRIORITY ANTIRETROVIRALS FOR THE MEDICINES PATENT POOL Third Edition | December 2013 Advancing innovation, access, and public health 1 CONTENTS 3 Introduction 4 Section 1: ARV Prioritisation in Light of New WHO Treatment Guidelines 5 Priority Regimens for Adults 9 Priority Regimens for Children and Adolescents 13 Section 2: Prioritisation of New ARVs and ARVs in the Pipeline 16 Conclusions 18 Annex I - Methodology 22 Annex II - Product Cards 23 Annex IIa: Product Cards for ARVs Prioritised in Light of New WHO Treatment Guidelines 36 Annex IIb: Product Cards for New ARVs and ARVs in the Pipeline 45 Annex IIc: Products in Early Stages of Development* 48 Annex III - Acronyms and Definitions 51 References ACKNOWLEDGMENTS The lead authors of this Working Paper were Esteban Burrone, Head of Policy, and Fernando Pascual, Medical/Pharmaceutical Consultant, of the Medicines Patent Pool. The paper also benefitted from extensive inputs from Robyn Harshaw, Sandeep Juneja, Kaitlin Mara, Chan Park, and Greg Perry. The Medicines Patent Pool (MPP) wants to thank the following external peer reviewers for their comments on an earlier draft: Jintanat Ananworanich, Pascale Boulet, Pedro Cahn, Polly Clayden, Jennifer Cohn, Meg Doherty, Diane Gibb, Raul Gonzalez, Andy Gray, Andrew Hill, Janice Lee, Rohit Malpani, Carmen Pérez-Casas, David Ripin, Marco Vitoria, and the MPP Expert Advisory Group composed of: Labeeb Abboud, Jonathan Berger, Alexandra Calmy, Shing Chang, Carlos Correa, Nelson Juma Otwoma, Eun-Joo Min, Lita Nelsen, Achal Prabhala, Gracia Violeta Ross, Maximiliano Santa Cruz, and Wim Vandevelde. 2 INTRODUCTION TO THE THIRD EDITION OF THE WORKING PAPER Since the first antiretroviral (ARV) information on the patent status, regulatory status received regulatory approval by the US and market trends for different ARVs. -

Antiviral Drugs in the Treatment of Aids: What Is in the Pipeline ?
October 15, 2007 EU RO PE AN JOUR NAL OF MED I CAL RE SEARCH 483 Eur J Med Res (2007) 12: 483-495 © I. Holzapfel Publishers 2007 ANTIVIRAL DRUGS IN THE TREATMENT OF AIDS: WHAT IS IN THE PIPELINE ? Hans-Jürgen Stellbrink Infektionsmedizinisches Centrum Hamburg ICH, Hamburg, Germany Abstract even targeting immune responses have to be devel- Drug development in the field of HIV treatment is oped. Continued drug development serves the ulti- rapid. New nucleoside analogues (NRTI), non-nucleo- mate goal of normalization of life expectancy. side analogue reverse transcriptase inhibitors (NNR- TI), and protease inhibitors (PI) are currently being in- METHODS vestigated in human trials. Furthermore, inhibitors of HIV attachment, fusion and integrase with novel Drug development in the field of HIV infection is modes of action are being developed, which offer new highly competitive, and a company’s decision to pur- perspectives for the goal of a normalization of life-ex- sue or discontinue the development of a drug is dri- pectancy in HIV-infected individuals. The most ad- ven by economic rather than scientific considerations. vanced compounds likely to become licensed soon in- Of all candidate compounds, only a few reach the lev- clude the NNRTIs rilpivirine and etravirine, the inte- el of trials in humans, and some exhibit lack of effica- grase inhibitors raltegravir and elvitegravir, and mar- cy or toxicity problems at this stage. Some compounds aviroc and vicriviroc, novel inhibitors of the CCR5 also have no obvious advantage over currently avail- chemokine receptor, which functions as the major able ones, so that their development is discontinued.