Clinical Pharmacology Related Considerations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download Article PDF/Slides
Kan Lu, PharmD New Antiretrovirals for Based on a presentation at prn by Roy M. Gulick, md, mph the Treatment of HIV: Kan Lu, PharmD | Drug Development Fellow University of North Carolina School of Pharmacy Chapel Hill, North Carolina The View in 2006 Roy M. Gulick, md, mph Reprinted from The prn Notebook® | october 2006 | Dr. James F. Braun, Editor-in-Chief Director, Cornell Clinical Trials Unit | Associate Professor of Medicine, Meri D. Pozo, PhD, Managing Editor. Published in New York City by the Physicians’ Research Network, Inc.® Weill Medical College of Cornell University | New York, New York John Graham Brown, Executive Director. For further information and other articles available online, visit http://www.prn.org | All rights reserved. ©october 2006 substantial progress continues to be made in the arena of cokinetics and a long extracellular half-life of approximately 10 hours antiretroviral drug development. prn is again proud to present its annual (Zhu, 2003). During apricitabine’s development, a serious drug interac- review of the experimental agents to watch for in the coming months and tion with lamivudine (Epivir) was noted. Although the plasma years. This year’s review is based on a lecture by Dr. Roy M. Gulick, a long- concentrations of apricitabine were unaffected by coadministration of time friend of prn, and no stranger to the antiretroviral development lamivudine, the intracellular concentrations of apricitabine were reduced pipeline. by approximately sixfold. Additionally, the 50% inhibitory concentration To date, twenty-two antiretrovirals have been approved by the Food (ic50) of apricitabine against hiv with the M184V mutation was increased and Drug Administration (fda) for the treatment of hiv infection. -

Ep 2531027 B1
(19) TZZ ¥_Z _T (11) EP 2 531 027 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 31/4985 (2006.01) A61K 31/52 (2006.01) 06.05.2015 Bulletin 2015/19 A61K 31/536 (2006.01) A61K 31/513 (2006.01) A61K 38/55 (2006.01) A61P 31/18 (2006.01) (21) Application number: 11737484.3 (86) International application number: (22) Date of filing: 24.01.2011 PCT/US2011/022219 (87) International publication number: WO 2011/094150 (04.08.2011 Gazette 2011/31) (54) Therapeutic combination comprising dolutegravir, abacavir and lamivudine Therapeutische Zusammensetzung enthaltend Dolutegravir, Abacavir und Lamivudine Combinaison thérapeutique comprenant du dolutégravir, de l’abacavir et de la lamivudine (84) Designated Contracting States: (74) Representative: Gladwin, Amanda Rachel AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GlaxoSmithKline GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Global Patents (CN925.1) PL PT RO RS SE SI SK SM TR 980 Great West Road Designated Extension States: Brentford, Middlesex TW8 9GS (GB) BA ME (56) References cited: (30) Priority: 27.01.2010 US 298589 P WO-A1-2010/011812 WO-A2-2009/148600 US-A1- 2006 084 627 US-A1- 2006 084 627 (43) Date of publication of application: US-A1- 2008 076 738 US-A1- 2009 318 421 12.12.2012 Bulletin 2012/50 US-A1- 2009 318 421 US-B1- 6 544 961 (73) Proprietor: VIIV Healthcare Company • SONG1 et al: "The Effect of Ritonavir-Boosted Research Triangle Park, NC 27709 (US) ProteaseInhibitors on the HIV Integrase Inhibitor, S/GSK1349572,in Healthy Subjects", INTERNET , (72) Inventor: UNDERWOOD, Mark, Richard 15 September 2009 (2009-09-15), XP002697436, Research Triangle Park Retrieved from the Internet: URL:http: North Carolina 27709 (US) //www.natap.org/2009/ICCAC/ICCAC_ 52.htm [retrieved on 2013-05-21] Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -

The Design, Synthesis and Optimization of Allosteric Hiv-1
THE DESIGN, SYNTHESIS AND OPTIMIZATION OF ALLOSTERIC HIV-1 INTEGRASE INHIBITORS DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Janet Antwi Graduate Program in Pharmaceutical Sciences The Ohio State University 2017 Dissertation Committee: Professor James R. Fuchs, Advisor Professor Werner Tjarks Professor Karl A. Werbovetz Copyright by Janet Antwi 2017 Abstract In the past quarter century, there has been tremendous progress in the discovery of antiretroviral therapy, making HIV/AIDS a manageable chronic disease. However, the HIV virus is relentless and continues to evolve under drug pressure to escape control and continue infection. The enzyme HIV integrase is responsible for the incorporation of viral double stranded DNA into a host chromosomal DNA and has recently become an attractive target in combating HIV resistance. Raltegravir (RAL), elvitegravir (EVG) and dolutegravir (DTG) are three clinically approved active-site integrase inhibitors. Unfortunately, mutations of the enzyme observed in patients have resulted in resistance to thedrug in the clinic. A new approach to targeting integrase (IN) is the development of allosteric inhibitors that specifically target the protein-protein interaction between IN and its cellular cofactor LEDGF/p75. Recently discovered quinoline-based allosteric integrase inhibitor (ALLINI) B1224436 was the first compound to advance into clinical trials but was discontinued due to poor pharmacokinetic properties including low in vivo clearance. In addition, several reports have revealed the emergence of resistance due to mutation to quinoline based ALLINIs. Applying scaffold hopping approach, several pyridine-based, thiophenes, ii pyrazoles, isoquinolines and other heteroaromatic cores have been studied as ALLINIs. -

Trends in Antiretroviral Treatment in Australia
AUSTRALIAN HIV OBSERVATIONAL DATABASE (AHOD) ANNUAL REPORT (Volume 20, Number 1: December 2020) 2020 Clinical characteristics of overseas-born men who have sex with men (MSM) in the AHOD cohort and implications for clinical practice In Australia HIV notifications are increasing among overseas-born men who have sex with men (MSM), particularly among Asian-born MSM. Australian evidence suggests that culturally and/or linguistically diverse populations are less likely to start treatment early irrespective of CD4 cell count at diagnosis, but little is known about response once in care. Using data from AHOD, Jolie L Hutchinson and colleagues (2020) compared treatment response in overseas-born MSM from non-English-speaking countries with Australian-born MSM, further categorised based on participation in the Australian Temporary Residents Access Study (ATRAS) which provide temporary residents ineligible for Medicare, access to HIV treatment. ATRAS patients were chosen as the closest surrogate to identifying newly arrived overseas-born MSM. The authors explored the time to first virological suppression (VS) (viral load (VL) <400 copies/mL) and time to virological failure (VF) (>400 copies/mL after suppression). CD4 cell counts and VL measurements were taken at treatment initiation. Adjusted Hazard Ratios (HR) are reported with 95% CI. Results, as shown in figure 1, indicate that overseas-born MSM did not differ significantly in the rate of VS or in the rate of first VF after suppression. This result is different from findings in other settings, and differences may, in part, be explained by the nature of healthcare provision. In Australia, all residents can access ART for free or with a small co-payment; those ineligible for Medicare can get pharmaceutical company-provided ART which is not necessarily straightforward for non-English speakers. -

HIV/AIDS Technologies: a Review of Progress to Date and Current Prospects
Working Paper No.6 HIV/AIDS Technologies: A review of progress to date and current prospects COMMISSIONED BY: aids2031 Science and Technology Working Group AUTHORED BY: KEITH ALCORN NAM Publications Disclaimer: The views expressed in this paper are those of the author(s) and do not necessarily reflect the official policy, position, or opinions of the wider aids2031 initiative or partner organizations aids2031 Science and Technology working group A review of progress to date and current prospects October 2008 Acronyms 3TC lamivudine ANRS Agènce Nationale de Récherche sur la Sida ART Antiretroviral therapy ARV Antiretroviral AZT azidothymidine or zidovudine bDNA branched DNA CDC US Centers for Disease Control CHER Children with HIV Early Antiretroviral therapy (study) CTL Cytotoxic T-lymphocyte D4T stavudine DSMB Data and Safety Monitoring Board EFV Efavirenz ELISA Enzyme Linked Immunosorbent Assay FDC Fixed-dose combination FTC Emtricitabine HAART Highly Active Antiretroviral Therapy HBAC Home-based AIDS care HCV Hepatitis C virus HPTN HIV Prevention Trials Network HSV-2 Herpes simplex virus type 2 IAVI International AIDS Vaccine Initiative IL-2 Interleukin-2 LED Light-emitting diode LPV/r Lopinavir/ritonavir MIRA Methods for Improving Reproductive Health in Africa trial MSF Médecins sans Frontières MSM Men who have sex with men MVA Modified vaccinia Ankara NIH US National Institutes of Health NRTI Nucleoside reverse transcriptase inhibitor NNRTI Non-nucleoside reverse transcriptase inhibitor OBT Optimised background therapy PCR Polymerase -

The Future of HIV Prevention and Treatment for Youth #Strive2optimize
THE AMERICAN ACADEMY OF HIV MEDICINE www.aahivm.org DECEMBER 2019 Patient Care, Practice Management & Professional Development Information for HIV Care Providers HIVSpecialist Integrating Transgender Healthcare for 24 The Future of Adolescents Not Your Parents’ HIV Prevention and Sex Talks 30 Treatment for Youth Adapting the Clinical Response 34 Fear of Addressing Substance Use and 36 Addiction DURABLE POWER AT PRESCRIBED REGIMEN FOR HIV-1 TREATMENT WEEK 144 Source: Ipsos Healthcare US HIV Therapy Monitor & Scope Study May-July 2019. BIKTARVY® (bictegravir 50 mg, emtricitabine 200 mg, and tenofovir alafenamide 25 mg) combines the FTC/TAF* backbone with bictegravir, a novel and unboosted INSTI—for a powerful STR1,2 Learn more about the BIKTARVY 144 week data at BIKTARVY144.com Long-term e cacy in treatment-naïve adults at Week 1442-7 Results noninferior to comparators No treatment-emergent resistance associated with BIKTARVY through Week 1442-7 Study 1489: Virologic Response Study 1490: Virologic Response Week 48 Week 144 Week 48 Week 144 -0.6% (-4.8% to -2.6% (-8.5% to -3.5% (-7.9% to -1.9% (-7.8% to 1.0%; p=0.12)† 3.9%; p=0.52)† 100 3.6%; p=0.78)† 3.4%; p=0.39)† 100 HIV-1 RNA HIV-1 RNA <50 copies/mL <50 copies/mL % % % 92 93 % 93 80 % 80 89 % CASES 82% 84 82% 84 OF RESISTANCE WITH 60 60 BIKTARVY ve Adults, % Adults, ve ve Adults, % Adults, ve ï ï 0 40 40 In two large phase 3 clinical trials in treatment-naïve adults 20 20 • Among 634 treatment-naïve adults in Studies 1489 and 1490, 8 treatment-failure subjects were Treatment-Na Treatment-Na 0 0 tested and no amino acid substitutions emerged that were associated with BIKTARVY resistance Virologic failure Virologic failure HIV-1 RNA 1% 3% 1% 3% HIV-1 RNA 4% 1% 5% 3% ≥50 copies/mL ≥50 copies/mL BIKTARVY ABC/DTG/3TC BIKTARVY FTC/TAF+DTG urine glucose, and urine protein in all patients (n=314) (n=315) (n=320) (n=325) IMPORTANT SAFETY INFORMATION (cont’d) Contraindications as clinically appropriate. -

Synthesis and Development of Long-Acting Abacavir Prodrug Nanoformulations
University of Nebraska Medical Center DigitalCommons@UNMC Theses & Dissertations Graduate Studies Summer 8-19-2016 Synthesis and Development of Long-Acting Abacavir Prodrug Nanoformulations Dhirender Singh University of Nebraska Medical Center Follow this and additional works at: https://digitalcommons.unmc.edu/etd Part of the Pharmaceutical Preparations Commons, and the Virus Diseases Commons Recommended Citation Singh, Dhirender, "Synthesis and Development of Long-Acting Abacavir Prodrug Nanoformulations" (2016). Theses & Dissertations. 140. https://digitalcommons.unmc.edu/etd/140 This Dissertation is brought to you for free and open access by the Graduate Studies at DigitalCommons@UNMC. It has been accepted for inclusion in Theses & Dissertations by an authorized administrator of DigitalCommons@UNMC. For more information, please contact [email protected]. SYNTHESIS AND DEVELOPMENT OF LONG-ACTING ABACAVIR PRODRUG NANOFORMULATIONS by Dhirender Singh A DISSERTATION Presented to the Faculty of the Graduate School in the University of Nebraska Medical Center in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Department of Pharmaceutical Science Under the Supervision of Dr. Howard E. Gendelman University of Nebraska Medical Center, Omaha, Nebraska August 2016 Supervisory Committee: Howard E. Gendelman, M.D. Ram Mahato, Ph.D JoEllyn M. McMillan, Ph.D. David Oupicky, Ph.D SYNTHESIS AND DEVELOPMENT OF LONG-ACTING ABACAVIR PRODRUG NANOFORMULATIONS Dhirender Singh, Ph.D. University of Nebraska Medical Center, 2016 Supervisor: Howard E Gendelman, M.D. Over the past decade, work from our laboratory has demonstrated the potential of targeted nanoformulated antiretroviral therapy (nanoART) to produce sustained high plasma and tissue drug concentrations for weeks following a single intramuscular (IM) administration that can suppress ongoing viral replication and mitigate dose associated viral resistance. -

Mutational Studies of Novel Screened Molecules Against Wild and Mutated HIV-1 Integrase Using Molecular Docking Studies Pawan Gupta1,2*, Prabha Garg2
Research Article Mutational studies of novel screened molecules against wild and mutated HIV-1 integrase using molecular docking studies Pawan Gupta1,2*, Prabha Garg2 ABSTRACT Background and Aim: The screened molecules which proposed novel HIV-1 integrase inhibitors were collected from the literature. Mutational studies were performed to check whether these molecules are having good binding affinity against mutated HIV-1 IN or not using molecular docking technique. Materials and Methods: First, homology models of the mutated HIV-1 IN were prepared and subsequently all the models were refined and optimized in MODELLER program. Next, molecular docking studies were performed into the active site of mutated HIV-1 IN models using the proposed inhibitors in AutoDock 4.1 program. The results of these studies were compared with the wild type docking studies. Results: The docking studies were found that some of the screened molecules (ZINC1245110, 131614, 92749, ZINC05181828, and ZINC13147504) followed the same binding patterns (in term of locations, interactions, and binding score) as found with wild type HIV-1 IN. Conclusions: Computationally, the same binding patterns were exhibited by these molecules (ZINC1245110, 131614, 92749, ZINC05181828, and ZINC13147504) against mutated models as wild type. This elucidated that these molecules having susceptibility against the drug-resistant HIV-1 IN. Hence, these molecules may be used as a starting point to design novel inhibitors against mutated HIV-1 IN, which need to be confirmed experimentally. KEY WORDS: Docking, Drug resistance, Homology modeling, HIV-1 integrase, Mutation INTRODUCTION Drug resistance is the inevitable consequence of incomplete suppression of HIV-1 replication. The Human immuno-virus (HIV) causes AIDS. -

Bictegravir-Tenofovir Alafenamide-Emtricitabine (Biktarvy)
© National HIV Curriculum PDF created September 25, 2021, 12:10 pm Bictegravir-Tenofovir alafenamide-Emtricitabine (Biktarvy) Table of Contents Bictegravir-Tenofovir alafenamide-Emtricitabine Biktarvy Summary Drug Summary Key Clinical Trials Key Drug Interactions Figures Drug Summary Bictegravir-tenofovir alafenamide-emtricitabine is a single-tablet regimen that is comprised of an integrase strand transfer inhibitor (bictegravir) combined with two nucleoside reverse transcriptase inhibitors (tenofovir alafenamide and emtricitabine). Bictegravir, which is not FDA-approved as an individual antiretroviral medication, has a high barrier to resistance, is well tolerated, and, extrapolating from the phase 3 data, has virologic efficacy comparable to dolutegravir. Bictegravir levels can be reduced with medications or oral supplement that contain polyvalent cations (e.g. Ca, Mg, Al, Fe). Bictegravir blocks tubular secretion of creatinine and typically raises serum creatinine by about 0.1 mg/dL, but without affecting renal glomerular function. Phase 3 trials in antiretroviral-naïve persons as well as switch trials in persons with virologic suppression have shown excellent efficacy with bictegravir-tenofovir alafenamide-emtricitabine. Available data suggest that bictegravir has a high genetic barrier to resistance. Key Clinical Trials In a phase 3 trial, 629 antiretroviral-naïve adults were randomized to receive bictegravir-tenofovir alafenamide-emtricitabine or dolutegravir-abacavir-lamivudine; after 48 weeks, there was no significant difference -

Guidelines for the Use of Antiretroviral Agents in Adults and Adolescent Living With
Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV Developed by the DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents – A Working Group of the Office of AIDS Research Advisory Council (OARAC) How to Cite the Adult and Adolescent Guidelines: Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. Available at https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/ AdultandAdolescentGL.pdf. Accessed [insert date] [insert page number, table number, etc. if applicable] It is emphasized that concepts relevant to HIV management evolve rapidly. The Panel has a mechanism to update recommendations on a regular basis, and the most recent information is available on the HIVinfo Web site (http://hivinfo.nih.gov). What’s New in the Guidelines? August 16, 2021 Hepatitis C Virus/HIV Coinfection • Table 18 of this section has been updated to include recommendations regarding concomitant use of fostemsavir or long acting cabotegravir plus rilpivirine with different hepatitis C treatment regimens. June 3, 2021 What to Start • Since the release of the last guidelines, updated data from the Botswana Tsepamo study have shown that the prevalence of neural tube defects (NTD) associated with dolutegravir (DTG) use during conception is much lower than previously reported. Based on these new data, the Panel now recommends that a DTG-based regimen can be prescribed for most people with HIV who are of childbearing potential. Before initiating a DTG-based regimen, clinicians should discuss the risks and benefits of using DTG with persons of childbearing potential, to allow them to make an informed decision. -
14-258 Phrma HIV/AIDS2014 0819.Indd
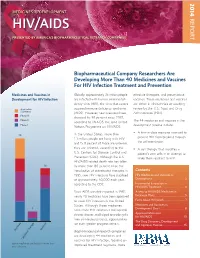
2014 MEDICINES IN DEVELOPMENT REPORT HIV/AIDS PRESENTED BY AMERICA’S BIOPHARMACEUTICAL RESEARCH COMPANIES Biopharmaceutical Company Researchers Are Developing More Than 40 Medicines and Vaccines For HIV Infection Treatment and Prevention Medicines and Vaccines in Globally, approximately 35 million people effective therapies, and preventative Development for HIV Infection are infected with human immunodefi - vaccines. These medicines and vaccines ciency virus (HIV), the virus that causes are either in clinical trials or awaiting Application acquired immune defi ciency syndrome review by the U.S. Food and Drug Submitted (AIDS). However, new infections have Administration (FDA). Phase III dropped by 38 percent since 2001, Phase II The 44 medicines and vaccines in the according to UNAIDS, the Joint United Phase I development pipeline include: Nations Programme on HIV/AIDS. • A fi rst-in-class medicine intended to In the United States, more than 25 prevent HIV from breaking through 1.1 million people are living with HIV the cell membrane. and 15.8 percent of those are unaware they are infected, according to the • A cell therapy that modifi es a U.S. Centers for Disease Control and patient’s own cells in an attempt to Prevention (CDC). Although the U.S. make them resistant to HIV. HIV/AIDS-related death rate has fallen 16 by more than 80 percent since the introduction of antiretroviral therapies in Contents 1995, new HIV infections have stabilized HIV Medicines and Vaccines in at approximately 50,000 each year, Development ......................................2 according to the CDC. Incremental Innovation in HIV/AIDS Treatment .......................... 4 Since AIDS was fi rst reported in 1981, Access to HIV/AIDS Medicines in nearly 40 medicines have been approved Exchange Plans ...................................5 to treat HIV infection in the United Facts About HIV/AIDS ........................7 States. -

Management of Hiv Infection
MANAGEMENT OF HIV INFECTION Federal Bureau of Prisons Clinical Guidance September 2018 Clinical guidance is made available to the public for informational purposes only. The Federal Bureau of Prisons (BOP) does not warrant this guidance for any other purpose, and assumes no responsibility for any injury or damage resulting from the reliance thereof. Proper medical practice necessitates that all cases are evaluated on an individual basis and that treatment decisions are patient-specific. Referenced Program Statement versions within this document are for informational purposes only. Please refer to the most current version of the referenced Program Statement. Consult the BOP Health Management Resources Web page to determine the date of the most recent update to this document: http://www.bop.gov/resources/health_care_mngmt.jsp Federal Bureau of Prisons Management of HIV Infection Clinical Guidance September 2018 WHAT’S NEW IN THIS DOCUMENT? This document updates the December 2017 version of the BOP Clinical Guidance on the Management of HIV Infection. Treatment information throughout this document was updated to be in line with the Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents issued by the Department of Health and Human Services (DHHS) in 2018. NOTEWORTHY CHANGES INCLUDE REVISIONS IN THE FOLLOWING AREAS: • INDICATIONS FOR HIV TESTING: An opt out strategy of voluntary testing for HIV infection is recommended for all inmates, regardless of sentencing status, including new intakes and those already established in the population who have not declined testing. • MENINGOCOCCAL VACCINE: Adults with HIV infection who have not been previously vaccinated should receive a 2-dose primary series of MenACWY (MCV4), available as Menactra® or Menveo®, at least 2 months apart, and then revaccinated every 5 years.