Peroxidase 1 Peroxidase
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Independent Evolution of Four Heme Peroxidase Superfamilies
Archives of Biochemistry and Biophysics xxx (2015) xxx–xxx Contents lists available at ScienceDirect Archives of Biochemistry and Biophysics journal homepage: www.elsevier.com/locate/yabbi Independent evolution of four heme peroxidase superfamilies ⇑ Marcel Zámocky´ a,b, , Stefan Hofbauer a,c, Irene Schaffner a, Bernhard Gasselhuber a, Andrea Nicolussi a, Monika Soudi a, Katharina F. Pirker a, Paul G. Furtmüller a, Christian Obinger a a Department of Chemistry, Division of Biochemistry, VIBT – Vienna Institute of BioTechnology, University of Natural Resources and Life Sciences, Muthgasse 18, A-1190 Vienna, Austria b Institute of Molecular Biology, Slovak Academy of Sciences, Dúbravská cesta 21, SK-84551 Bratislava, Slovakia c Department for Structural and Computational Biology, Max F. Perutz Laboratories, University of Vienna, A-1030 Vienna, Austria article info abstract Article history: Four heme peroxidase superfamilies (peroxidase–catalase, peroxidase–cyclooxygenase, peroxidase–chlo- Received 26 November 2014 rite dismutase and peroxidase–peroxygenase superfamily) arose independently during evolution, which and in revised form 23 December 2014 differ in overall fold, active site architecture and enzymatic activities. The redox cofactor is heme b or Available online xxxx posttranslationally modified heme that is ligated by either histidine or cysteine. Heme peroxidases are found in all kingdoms of life and typically catalyze the one- and two-electron oxidation of a myriad of Keywords: organic and inorganic substrates. In addition to this peroxidatic activity distinct (sub)families show pro- Heme peroxidase nounced catalase, cyclooxygenase, chlorite dismutase or peroxygenase activities. Here we describe the Peroxidase–catalase superfamily phylogeny of these four superfamilies and present the most important sequence signatures and active Peroxidase–cyclooxygenase superfamily Peroxidase–chlorite dismutase superfamily site architectures. -

SUPPLEMENTARY DATA Supplementary Figure 1. The
SUPPLEMENTARY DATA Supplementary Figure 1. The results of Sirt1 activation in primary cultured TG cells using adenoviral system. GFP expression served as the control (n = 4 per group). Supplementary Figure 2. Two different Sirt1 activators, SRT1720 (0.5 µM or 1 µM ) and RSV (1µM or 10µM), induced the upregulation of Sirt1 in the primary cultured TG cells (n = 4 per group). ©2016 American Diabetes Association. Published online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-1283/-/DC1 SUPPLEMENTARY DATA Supplementary Table 1. Primers used in qPCR Gene Name Primer Sequences Product Size (bp) Sirt1 F: tgccatcatgaagccagaga 241 (NM_001159589) R: aacatcgcagtctccaagga NOX4 F: tgtgcctttattgtgcggag 172 (NM_001285833.1) R: gctgatacactggggcaatg Supplementary Table 2. Antibodies used in Western blot or Immunofluorescence Antibody Company Cat. No Isotype Dilution Sirt1 Santa Cruz * sc-15404 Rabbit IgG 1/200 NF200 Sigma** N5389 Mouse IgG 1/500 Tubulin R&D# MAB1195 Mouse IgG 1/500 NOX4 Abcam† Ab133303 Rabbit IgG 1/500 NOX2 Abcam Ab129068 Rabbit IgG 1/500 phospho-AKT CST‡ #4060 Rabbit IgG 1/500 EGFR CST #4267 Rabbit IgG 1/500 Ki67 Santa Cruz sc-7846 Goat IgG 1/500 * Santa Cruz Biotechnology, Santa Cruz, CA, USA ** Sigma aldrich, Shanghai, China # R&D Systems Inc, Minneapolis, MN, USA † Abcam, Inc., Cambridge, MA, USA ‡ Cell Signaling Technology, Inc., Danvers, MA, USA ©2016 American Diabetes Association. Published online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-1283/-/DC1 SUPPLEMENTARY DATA Supplementary -

GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis Giovanni C
REVIEW GPX4 www.proteomics-journal.com GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis Giovanni C. Forcina and Scott J. Dixon* formation of toxic radicals (e.g., R-O•).[5] Oxygen is necessary for aerobic metabolism but can cause the harmful The eight mammalian GPX proteins fall oxidation of lipids and other macromolecules. Oxidation of cholesterol and into three clades based on amino acid phospholipids containing polyunsaturated fatty acyl chains can lead to lipid sequence similarity: GPX1 and GPX2; peroxidation, membrane damage, and cell death. Lipid hydroperoxides are key GPX3, GPX5, and GPX6; and GPX4, GPX7, and GPX8.[6] GPX1–4 and 6 (in intermediates in the process of lipid peroxidation. The lipid hydroperoxidase humans) are selenoproteins that contain glutathione peroxidase 4 (GPX4) converts lipid hydroperoxides to lipid an essential selenocysteine in the enzyme + alcohols, and this process prevents the iron (Fe2 )-dependent formation of active site, while GPX5, 6 (in mouse and toxic lipid reactive oxygen species (ROS). Inhibition of GPX4 function leads to rats), 7, and 8 use an active site cysteine lipid peroxidation and can result in the induction of ferroptosis, an instead. Unlike other family members, GPX4 (PHGPx) can act as a phospholipid iron-dependent, non-apoptotic form of cell death. This review describes the hydroperoxidase to reduce lipid perox- formation of reactive lipid species, the function of GPX4 in preventing ides to lipid alcohols.[7,8] Thus,GPX4ac- oxidative lipid damage, and the link between GPX4 dysfunction, lipid tivity is essential to maintain lipid home- oxidation, and the induction of ferroptosis. ostasis in the cell, prevent the accumula- tion of toxic lipid ROS and thereby block the onset of an oxidative, iron-dependent, non-apoptotic mode of cell death termed 1. -

Prokaryotic Origins of the Non-Animal Peroxidase Superfamily and Organelle-Mediated Transmission to Eukaryotes
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Genomics 89 (2007) 567–579 www.elsevier.com/locate/ygeno Prokaryotic origins of the non-animal peroxidase superfamily and organelle-mediated transmission to eukaryotes Filippo Passardi a, Nenad Bakalovic a, Felipe Karam Teixeira b, Marcia Margis-Pinheiro b,c, ⁎ Claude Penel a, Christophe Dunand a, a Laboratory of Plant Physiology, University of Geneva, Quai Ernest-Ansermet 30, CH-1211 Geneva 4, Switzerland b Department of Genetics, Institute of Biology, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil c Department of Genetics, Federal University of Rio Grande do Sul, Rio Grande do Sul, Brazil Received 16 June 2006; accepted 18 January 2007 Available online 13 March 2007 Abstract Members of the superfamily of plant, fungal, and bacterial peroxidases are known to be present in a wide variety of living organisms. Extensive searching within sequencing projects identified organisms containing sequences of this superfamily. Class I peroxidases, cytochrome c peroxidase (CcP), ascorbate peroxidase (APx), and catalase peroxidase (CP), are known to be present in bacteria, fungi, and plants, but have now been found in various protists. CcP sequences were detected in most mitochondria-possessing organisms except for green plants, which possess only ascorbate peroxidases. APx sequences had previously been observed only in green plants but were also found in chloroplastic protists, which acquired chloroplasts by secondary endosymbiosis. CP sequences that are known to be present in prokaryotes and in Ascomycetes were also detected in some Basidiomycetes and occasionally in some protists. -

Extracellular Vesicles Derived from Induced Pluripotent Stem Cells Promote Renoprotection in Acute Kidney Injury Model
cells Article Extracellular Vesicles Derived from Induced Pluripotent Stem Cells Promote Renoprotection in Acute Kidney Injury Model Federica Collino 1,2,3 , Jarlene A. Lopes 1,2,4, Marta Tapparo 5, Giovane G. Tortelote 1,6, Taís H. Kasai-Brunswick 1,2,4, Gustavo M.C. Lopes 1,2,4, Douglas B. Almeida 1,2,4, Renata Skovronova 7, Camila H. C. Wendt 1, Kildare R. de Miranda 1,4,8, Benedetta Bussolati 7 , Adalberto Vieyra 1,2,4,9,* and Rafael Soares Lindoso 1,2,4,* 1 Institute of Biophysics Carlos Chagas Filho, Federal University of Rio de Janeiro, 21941-902 Rio de Janeiro, Brazil; [email protected] (F.C.); [email protected] (J.A.L.); [email protected] (G.G.T.); [email protected] (T.H.K.-B.); [email protected] (G.M.C.L.); [email protected] (D.B.A.); [email protected] (C.H.C.W.); [email protected] (K.R.d.M.) 2 National Institute of Science and Technology for Regenerative Medicine-REGENERA, Federal University of Rio de Janeiro, 21941-902 Rio de Janeiro, Brazil 3 Department of Biomedical Sciences, University of Padova, 35131 Padua, Italy 4 National Center for Structural Biology and Bioimaging/CENABIO, Federal University of Rio de Janeiro, 21941-902 Rio de Janeiro, Brazil 5 Department of Medical Sciences, Molecular Biotechnology Center, University of Torino, 10126 Torino, Italy; [email protected] 6 Department of Pediatrics’ Section of Pediatric Nephrology, Tulane University School of Medicine, New Orleans, LA 70112, USA 7 Department of Molecular Biotechnology and Health Sciences, University of Torino, -

New Automatically Built Profiles for a Better Understanding of the Peroxidase Superfamily Evolution
University of Geneva Practical training report submitted for the Master Degree in Proteomics and Bioinformatics New automatically built profiles for a better understanding of the peroxidase superfamily evolution presented by Dominique Koua Supervisors: Dr Christophe DUNAND Dr Nicolas HULO Laboratory of Plant Physiology, Dr Christian J.A. SIGRIST University of Geneva Swiss Institute of Bioinformatics PROSITE group. Geneva, April, 18th 2008 Abstract Motivation: Peroxidases (EC 1.11.1.x), which are encoded by small or large multigenic families, are involved in several important physiological and developmental processes. These proteins are extremely widespread and present in almost all living organisms. An important number of haem and non-haem peroxidase sequences are annotated and classified in the peroxidase database PeroxiBase (http://peroxibase.isb-sib.ch). PeroxiBase contains about 5800 peroxidase sequences classified as haem peroxidases and non-haem peroxidases and distributed between thirteen superfamilies and fifty subfamilies, (Passardi et al., 2007). However, only a few classification tools are available for the characterisation of peroxidase sequences: InterPro motifs, PRINTS and specifically designed PROSITE profiles. However, these PROSITE profiles are very global and do not allow the differenciation between very close subfamily sequences nor do they allow the prediction of specific cellular localisations. Due to the rapid growth in the number of available sequences, there is a need for continual updates and corrections of peroxidase protein sequences as well as for new tools that facilitate acquisition and classification of existing and new sequences. Currently, the PROSITE generalised profile building manner and their usage do not allow the differentiation of sequences from subfamilies showing a high degree of similarity. -
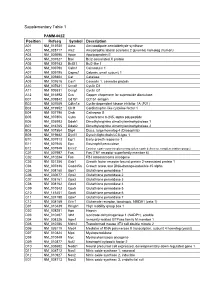
Supplementary Table 1
Supplementary Table 1 PAMM-062Z Position Refseq Symbol Description A01 NM_013930 Aass Aminoadipate-semialdehyde synthase A02 NM_028717 Als2 Amyotrophic lateral sclerosis 2 (juvenile) homolog (human) A03 NM_009696 Apoe Apolipoprotein E A04 NM_007527 Bax Bcl2-associated X protein A05 NM_009743 Bcl2l1 Bcl2-like 1 A06 NM_009790 Calm1 Calmodulin 1 A07 NM_009795 Capns1 Calpain, small subunit 1 A08 NM_009804 Cat Catalase A09 NM_007616 Cav1 Caveolin 1, caveolae protein A10 NM_007631 Ccnd1 Cyclin D1 A11 NM_009831 Ccng1 Cyclin G1 A12 NM_016892 Ccs Copper chaperone for superoxide dismutase B01 NM_009842 Cd151 CD151 antigen B02 NM_007669 Cdkn1a Cyclin-dependent kinase inhibitor 1A (P21) B03 NM_019952 Clcf1 Cardiotrophin-like cytokine factor 1 B04 NM_007798 Ctsb Cathepsin B B05 NM_007806 Cyba Cytochrome b-245, alpha polypeptide B06 NM_026993 Ddah1 Dimethylarginine dimethylaminohydrolase 1 B07 NM_016765 Ddah2 Dimethylarginine dimethylaminohydrolase 2 B08 NM_007864 Dlg4 Discs, large homolog 4 (Drosophila) B09 NM_019682 Dynll1 Dynein light chain LC8-type 1 B10 NM_007913 Egr1 Early growth response 1 B11 NM_007946 Epx Eosinophil peroxidase B12 NM_007949 Ercc2 Excision repair cross-complementing rodent repair deficiency, complementation group 2 C01 NM_007987 Fas Fas (TNF receptor superfamily member 6) C02 NM_010234 Fos FBJ osteosarcoma oncogene C03 NM_021356 Gab1 Growth factor receptor bound protein 2-associated protein 1 C04 NM_007836 Gadd45a Growth arrest and DNA-damage-inducible 45 alpha C05 NM_008160 Gpx1 Glutathione peroxidase 1 C06 NM_030677 Gpx2 Glutathione -

Catalysis of Peroxide Reduction by Fast Reacting Protein Thiols Focus Review †,‡ †,‡ ‡,§ ‡,§ ∥ Ari Zeida, Madia Trujillo, Gerardo Ferrer-Sueta, Ana Denicola, Darío A
Review Cite This: Chem. Rev. 2019, 119, 10829−10855 pubs.acs.org/CR Catalysis of Peroxide Reduction by Fast Reacting Protein Thiols Focus Review †,‡ †,‡ ‡,§ ‡,§ ∥ Ari Zeida, Madia Trujillo, Gerardo Ferrer-Sueta, Ana Denicola, Darío A. Estrin, and Rafael Radi*,†,‡ † ‡ § Departamento de Bioquímica, Centro de Investigaciones Biomedicaś (CEINBIO), Facultad de Medicina, and Laboratorio de Fisicoquímica Biologica,́ Facultad de Ciencias, Universidad de la Republica,́ 11800 Montevideo, Uruguay ∥ Departamento de Química Inorganica,́ Analítica y Química-Física and INQUIMAE-CONICET, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, 2160 Buenos Aires, Argentina ABSTRACT: Life on Earth evolved in the presence of hydrogen peroxide, and other peroxides also emerged before and with the rise of aerobic metabolism. They were considered only as toxic byproducts for many years. Nowadays, peroxides are also regarded as metabolic products that play essential physiological cellular roles. Organisms have developed efficient mechanisms to metabolize peroxides, mostly based on two kinds of redox chemistry, catalases/peroxidases that depend on the heme prosthetic group to afford peroxide reduction and thiol-based peroxidases that support their redox activities on specialized fast reacting cysteine/selenocysteine (Cys/Sec) residues. Among the last group, glutathione peroxidases (GPxs) and peroxiredoxins (Prxs) are the most widespread and abundant families, and they are the leitmotif of this review. After presenting the properties and roles of different peroxides in biology, we discuss the chemical mechanisms of peroxide reduction by low molecular weight thiols, Prxs, GPxs, and other thiol-based peroxidases. Special attention is paid to the catalytic properties of Prxs and also to the importance and comparative outlook of the properties of Sec and its role in GPxs. -

Glutathione Peroxidase 3 (GPX3) (102-114) Goat Polyclonal Antibody Product Data
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for AP22523PU-N Glutathione Peroxidase 3 (GPX3) (102-114) Goat Polyclonal Antibody Product data: Product Type: Primary Antibodies Applications: ELISA, IHC, WB Recommended Dilution: ELISA: 1/1000. Immunohistochemistry on Paraffin Sections: 5 µg/ml. Western Blot: 1 - 3 µg/ml. Reactivity: Bat, Bovine, Canine, Equine, Human, Monkey, Mouse, Porcine, Rat Host: Goat Clonality: Polyclonal Immunogen: Synthetic peptide from internal region of human GPX3 Specificity: This antibody detects Glutathione peroxidase 3 at aa 102-114. It may cross-react with epididymis-specific GPX5 and olfactory epithelium-speciific GPX6. Formulation: Tris saline buffer, pH 7.3, 0.5% BSA, 0.02% sodium azide State: Liquid Ig fraction Concentration: lot specific Purification: Immunoaffinity chromatography Conjugation: Unconjugated Storage: Store at 2 - 8 °C for up to three months or (in aliquots) at -20 °C for longer. Avoid repeated freezing and thawing. Stability: Shelf life: one year from despatch. Gene Name: Homo sapiens glutathione peroxidase 3 (GPX3), transcript variant 1 Database Link: Entrez Gene 14778 MouseEntrez Gene 64317 RatEntrez Gene 2878 Human P22352 Background: Glutathione peroxidase catalyzes the reduction of hydrogen peroxide, organic hydroperoxide, and lipid peroxides by reduced glutathione and functions in the protection of cells against oxidative damage. Human plasma glutathione peroxidase has been shown to be a selenium- containing enzyme. GPX3 expression appears to be tissue-specific. Synonyms: GPX-3, GPXP, GSHPx-3, GPx-3, GSHPx-P, GSHPXP, GPx-P, GPXP Protein Families: Druggable Genome, Secreted Protein This product is to be used for laboratory only. -

The Emerging Roles of Antioxidant Enzymes by Dietary Phytochemicals in Vascular Diseases
life Review The Emerging Roles of Antioxidant Enzymes by Dietary Phytochemicals in Vascular Diseases Seung Eun Lee and Yong Seek Park * Department of Microbiology, School of Medicine, Kyung Hee University, Seoul 02447, Korea; [email protected] * Correspondence: [email protected]; Tel.: +82-02-961-0267 Abstract: Vascular diseases are major causes of death worldwide, causing pathologies including diabetes, atherosclerosis, and chronic obstructive pulmonary disease (COPD). Exposure of the vascular system to a variety of stressors and inducers has been implicated in the development of various human diseases, including chronic inflammatory diseases. In the vascular wall, antioxidant enzymes form the first line of defense against oxidative stress. Recently, extensive research into the beneficial effects of phytochemicals has been conducted; phytochemicals are found in commonly used spices, fruits, and herbs, and are used to prevent various pathologic conditions, including vascular diseases. The present review aims to highlight the effects of dietary phytochemicals role on antioxidant enzymes in vascular diseases. Keywords: vascular diseases; antioxidant enzymes; bioactive compounds 1. Introduction Citation: Lee, S.E.; Park, Y.S. The Vascular diseases are responsible for numerous deaths annually worldwide. The Emerging Roles of Antioxidant pathogenesis of vascular disease involves the activation of pro-inflammatory signaling Enzymes by Dietary Phytochemicals pathways, expression of cytokines/chemokines, and elevated oxidative stress. Exposure in Vascular Diseases. Life 2021, 11, 199. https://doi.org/10.3390/ to oxidative stress may directly injure the vasculature and induce vascular dysfunction life11030199 by producing dysregulation of the immune response. Oxidative stress is caused by an imbalance between the production and accumulation of reactive oxygen species (ROS) and Academic Editor: Payaningal the capacity of antioxidant defense mechanisms favoring oxidants [1]. -

Characterization of Cytosolic Glutathione Peroxidase And
Aquatic Toxicology 130–131 (2013) 97–111 Contents lists available at SciVerse ScienceDirect Aquatic Toxicology jou rnal homepage: www.elsevier.com/locate/aquatox Characterization of cytosolic glutathione peroxidase and phospholipid-hydroperoxide glutathione peroxidase genes in rainbow trout (Oncorhynchus mykiss) and their modulation by in vitro selenium exposure a a b a d c a,∗ D. Pacitti , T. Wang , M.M. Page , S.A.M. Martin , J. Sweetman , J. Feldmann , C.J. Secombes a Scottish Fish Immunology Research Centre, Institute of Biological and Environmental Sciences, University of Aberdeen, Aberdeen AB24 2TZ, United Kingdom b Integrative and Environmental Physiology, Institute of Biological and Environmental Sciences, University of Aberdeen, Aberdeen AB24 2TZ, United Kingdom c Trace Element Speciation Laboratory, Department of Chemistry, University of Aberdeen, Aberdeen AB24 3UE, United Kingdom d Alltech Biosciences Centre, Sarney, Summerhill Rd, Dunboyne, Country Meath, Ireland a r t i c l e i n f o a b s t r a c t Article history: Selenium (Se) is an oligonutrient with both essential biological functions and recognized harmful effects. Received 4 July 2012 As the selenocysteine (SeCys) amino acid, selenium is integrated in several Se-containing proteins Received in revised form (selenoproteins), many of which are fundamental for cell homeostasis. Nevertheless, selenium may exert 19 December 2012 toxic effects at levels marginally above those required, mainly through the generation of reactive oxygen Accepted 20 December 2012 species (ROS). The selenium chemical speciation can strongly affect the bioavailability of this metal and its impact on metabolism, dictating the levels that can be beneficial or detrimental towards an organism. -

Involvement of Glutathione Peroxidases in the Occurrence And
Zhang et al. J Transl Med (2020) 18:247 https://doi.org/10.1186/s12967-020-02420-x Journal of Translational Medicine REVIEW Open Access Involvement of glutathione peroxidases in the occurrence and development of breast cancers Man‑Li Zhang1†, Hua‑Tao Wu2†, Wen‑Jia Chen1,3, Ya Xu1, Qian‑Qian Ye1,3, Jia‑Xin Shen4 and Jing Liu1,3* Abstract Glutathione peroxidases (GPxs) belong to a family of enzymes that is important in organisms; these enzymes pro‑ mote hydrogen peroxide metabolism and protect cell membrane structure and function from oxidative damage. Based on the establishment and development of the theory of the pathological roles of free radicals, the role of GPxs has gradually attracted researchers’ attention, and the involvement of GPxs in the occurrence and development of malignant tumors has been shown. On the other hand, the incidence of breast cancer in increasing, and breast cancer has become the leading cause of cancer‑related death in females worldwide; breast cancer is thought to be related to the increased production of reactive oxygen species, indicating the involvement of GPxs in these processes. Therefore, this article focused on the molecular mechanism and function of GPxs in the occurrence and development of breast cancer to understand their role in breast cancer and to provide a new theoretical basis for the treatment of breast cancer. Keywords: Glutathione peroxidase, Breast cancer, Reactive oxygen species, Occurrence Background [4]. However, the mechanisms of the occurrence, devel- Breast cancer has become the most common cancer and opment, and metastasis of breast cancer are very com- the leading cause of cancer-related deaths in females plicated and overlap, suggesting the necessity of diferent worldwide, according to a status report on the global therapies to treat diferent subtypes of breast cancer.