Unglycosylated Recombinant Human Glutathione Peroxidase 3 Mutant
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Role of Glutathionperoxidase 4 (GPX4) in Hematopoiesis and Leukemia
The role of glutathionperoxidase 4 (GPX4) in hematopoiesis and leukemia von Kira Célénie Stahnke Inaugral- Dissertation zur Erlangung der Doktorwürde der TierärztliChen Fakultät der Ludwig-Maximilians-Universität MünChen The role of glutathionperoxidase 4 (GPX4) in hematopoiesis and leukemia von Kira Célénie Stahnke aus Dortmund München 2016 Aus dem VeterinärwissensChaftliChen Department der TierärztliChen Fakultät der Ludwig-Maximilians-Universität München Lehrstuhl für Molekulare Tierzucht und Biotechnologie Arbeit angefertigt unter der Leitung von Univ.-Prof. Dr. Eckhard Wolf Angefertigt am Institut für Experimentelle TumorforsChung Universitätsklinikum Ulm Mentor: Prof. Dr. Christian Buske GedruCkt mit Genehmigung der TierärztliChen Fakultät der Ludwig-Maximilians-Universität München Dekan: Univ.-Prof. Dr. JoaChim Braun Berichterstatter: Univ.-Prof. Dr. Eckard Wolf Korreferent/en: Priv.-Doz. Dr. Bianka Schulz Tag der Promotion: 06.02.2016 Table of contents List of Abbreviations..............................................................................2 1 Introduction ........................................................................................6 1.1 ROS and oxidative stress in physiology and pathology..................................................... 6 1.1.1 SourCes of Reactive Oxygen SpeCies..................................................................................................6 1.1.2 PhysiologiCal role of ROS........................................................................................................................8 -

(ER) Membrane Contact Sites (MCS) Uses Toxic Waste to Deliver Messages Edgar Djaha Yoboue1, Roberto Sitia1 and Thomas Simmen2
Yoboue et al. Cell Death and Disease (2018) 9:331 DOI 10.1038/s41419-017-0033-4 Cell Death & Disease REVIEW ARTICLE Open Access Redox crosstalk at endoplasmic reticulum (ER) membrane contact sites (MCS) uses toxic waste to deliver messages Edgar Djaha Yoboue1, Roberto Sitia1 and Thomas Simmen2 Abstract Many cellular redox reactions housed within mitochondria, peroxisomes and the endoplasmic reticulum (ER) generate hydrogen peroxide (H2O2) and other reactive oxygen species (ROS). The contribution of each organelle to the total cellular ROS production is considerable, but varies between cell types and also over time. Redox-regulatory enzymes are thought to assemble at a “redox triangle” formed by mitochondria, peroxisomes and the ER, assembling “redoxosomes” that sense ROS accumulations and redox imbalances. The redoxosome enzymes use ROS, potentially toxic by-products made by some redoxosome members themselves, to transmit inter-compartmental signals via chemical modifications of downstream proteins and lipids. Interestingly, important components of the redoxosome are ER chaperones and oxidoreductases, identifying ER oxidative protein folding as a key ROS producer and controller of the tri-organellar membrane contact sites (MCS) formed at the redox triangle. At these MCS, ROS accumulations could directly facilitate inter-organellar signal transmission, using ROS transporters. In addition, ROS influence the flux 2+ 2+ of Ca ions, since many Ca handling proteins, including inositol 1,4,5 trisphosphate receptors (IP3Rs), SERCA pumps or regulators of the mitochondrial Ca2+ uniporter (MCU) are redox-sensitive. Fine-tuning of these redox and ion signaling pathways might be difficult in older organisms, suggesting a dysfunctional redox triangle may accompany 1234567890 1234567890 the aging process. -

A Machine Learning Tool for Predicting Protein Function Anna
The Woolf Classifier Building Pipeline: A Machine Learning Tool for Predicting Protein Function Anna Farrell-Sherman Submitted in Partial Fulfillment of the Prerequisite for Honors in Computer Science under the advisement of Eni Mustafaraj and Vanja Klepac-Ceraj May 2019 © 2019 Anna Farrell-Sherman Abstract Proteins are the machinery that allow cells to grow, reproduce, communicate, and create multicellular organisms. For all of their importance, scientists still have a hard time understanding the function of a protein based on its sequence alone. For my honors thesis in computer science, I created a machine learning tool that can predict the function of a protein based solely on its amino acid sequence. My tool gives scientists a structure in which to build a � Nearest Neighbor or random forest classifier to distinguish between proteins that can and cannot perform a given function. Using default Min-Max scaling, and the Matthews Correlation Coefficient for accuracy assessment, the Woolf Pipeline is built with simplified choices to guide users to success. i Acknowledgments There are so many people who made this thesis possible. First, thank you to my wonderful advisors, Eni and Vanja, who never gave up on me, and always pushed me to try my hardest. Thank you also to the other members of my committee, Sohie Lee, Shikha Singh, and Rosanna Hertz for supporting me through this process. To Kevin, Sophie R, Sophie E, and my sister Phoebe, thank you for reading my drafts, and advising me when the going got tough. To all my wonderful friends, who were always there with encouraging words, warm hugs, and many congratulations, I cannot thank you enough. -

SUPPLEMENTARY DATA Supplementary Figure 1. The
SUPPLEMENTARY DATA Supplementary Figure 1. The results of Sirt1 activation in primary cultured TG cells using adenoviral system. GFP expression served as the control (n = 4 per group). Supplementary Figure 2. Two different Sirt1 activators, SRT1720 (0.5 µM or 1 µM ) and RSV (1µM or 10µM), induced the upregulation of Sirt1 in the primary cultured TG cells (n = 4 per group). ©2016 American Diabetes Association. Published online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-1283/-/DC1 SUPPLEMENTARY DATA Supplementary Table 1. Primers used in qPCR Gene Name Primer Sequences Product Size (bp) Sirt1 F: tgccatcatgaagccagaga 241 (NM_001159589) R: aacatcgcagtctccaagga NOX4 F: tgtgcctttattgtgcggag 172 (NM_001285833.1) R: gctgatacactggggcaatg Supplementary Table 2. Antibodies used in Western blot or Immunofluorescence Antibody Company Cat. No Isotype Dilution Sirt1 Santa Cruz * sc-15404 Rabbit IgG 1/200 NF200 Sigma** N5389 Mouse IgG 1/500 Tubulin R&D# MAB1195 Mouse IgG 1/500 NOX4 Abcam† Ab133303 Rabbit IgG 1/500 NOX2 Abcam Ab129068 Rabbit IgG 1/500 phospho-AKT CST‡ #4060 Rabbit IgG 1/500 EGFR CST #4267 Rabbit IgG 1/500 Ki67 Santa Cruz sc-7846 Goat IgG 1/500 * Santa Cruz Biotechnology, Santa Cruz, CA, USA ** Sigma aldrich, Shanghai, China # R&D Systems Inc, Minneapolis, MN, USA † Abcam, Inc., Cambridge, MA, USA ‡ Cell Signaling Technology, Inc., Danvers, MA, USA ©2016 American Diabetes Association. Published online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-1283/-/DC1 SUPPLEMENTARY DATA Supplementary -

GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis Giovanni C
REVIEW GPX4 www.proteomics-journal.com GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis Giovanni C. Forcina and Scott J. Dixon* formation of toxic radicals (e.g., R-O•).[5] Oxygen is necessary for aerobic metabolism but can cause the harmful The eight mammalian GPX proteins fall oxidation of lipids and other macromolecules. Oxidation of cholesterol and into three clades based on amino acid phospholipids containing polyunsaturated fatty acyl chains can lead to lipid sequence similarity: GPX1 and GPX2; peroxidation, membrane damage, and cell death. Lipid hydroperoxides are key GPX3, GPX5, and GPX6; and GPX4, GPX7, and GPX8.[6] GPX1–4 and 6 (in intermediates in the process of lipid peroxidation. The lipid hydroperoxidase humans) are selenoproteins that contain glutathione peroxidase 4 (GPX4) converts lipid hydroperoxides to lipid an essential selenocysteine in the enzyme + alcohols, and this process prevents the iron (Fe2 )-dependent formation of active site, while GPX5, 6 (in mouse and toxic lipid reactive oxygen species (ROS). Inhibition of GPX4 function leads to rats), 7, and 8 use an active site cysteine lipid peroxidation and can result in the induction of ferroptosis, an instead. Unlike other family members, GPX4 (PHGPx) can act as a phospholipid iron-dependent, non-apoptotic form of cell death. This review describes the hydroperoxidase to reduce lipid perox- formation of reactive lipid species, the function of GPX4 in preventing ides to lipid alcohols.[7,8] Thus,GPX4ac- oxidative lipid damage, and the link between GPX4 dysfunction, lipid tivity is essential to maintain lipid home- oxidation, and the induction of ferroptosis. ostasis in the cell, prevent the accumula- tion of toxic lipid ROS and thereby block the onset of an oxidative, iron-dependent, non-apoptotic mode of cell death termed 1. -

Micrornas As New Regulators of Neutrophil Extracellular Trap Formation
International Journal of Molecular Sciences Review MicroRNAs as New Regulators of Neutrophil Extracellular Trap Formation Sonia Águila † , Ascensión M. de los Reyes-García †, María P. Fernández-Pérez, Laura Reguilón-Gallego, Laura Zapata-Martínez, Inmaculada Ruiz-Lorente, Vicente Vicente , Rocío González-Conejero *,‡ and Constantino Martínez *,‡ Department of Hematology and Medical Oncology, Morales Meseguer University Hospital, Centro Regional de Hemodonación, Universidad de Murcia, IMIB, C/Ronda de Garay S/N, 30003 Murcia, Spain; [email protected] (S.Á.); [email protected] (A.M.d.l.R.-G.); [email protected] (M.P.F.-P.); [email protected] (L.R.-G.); [email protected] (L.Z.-M.); [email protected] (I.R.-L.); [email protected] (V.V.) * Correspondence: [email protected] (R.G.-C.); [email protected] (C.M.); Tel.: +34-968341990 (R.G.-C. & C.M.); Fax: +34-968261914 (R.G.-C. & C.M.) † These authors contributed equally to this work. ‡ These authors shared senior authorship. Abstract: Neutrophil extracellular traps (NETs) are formed after neutrophils expelled their chromatin content in order to primarily capture and eliminate pathogens. However, given their characteristics due in part to DNA and different granular proteins, NETs may induce a procoagulant response linking inflammation and thrombosis. Unraveling NET formation molecular mechanisms as well Citation: Águila, S.; de los as the intracellular elements that regulate them is relevant not only for basic knowledge but also Reyes-García, A.M.; Fernández-Pérez, to design diagnostic and therapeutic tools that may prevent their deleterious effects observed in M.P.; Reguilón-Gallego, L.; several inflammatory pathologies (e.g., cardiovascular and autoimmune diseases, cancer). -

Role of Oxidative Stress and Nrf2/KEAP1 Signaling in Colorectal Cancer: Mechanisms and Therapeutic Perspectives with Phytochemicals
antioxidants Review Role of Oxidative Stress and Nrf2/KEAP1 Signaling in Colorectal Cancer: Mechanisms and Therapeutic Perspectives with Phytochemicals Da-Young Lee, Moon-Young Song and Eun-Hee Kim * College of Pharmacy and Institute of Pharmaceutical Sciences, CHA University, Seongnam 13488, Korea; [email protected] (D.-Y.L.); [email protected] (M.-Y.S.) * Correspondence: [email protected]; Tel.: +82-31-881-7179 Abstract: Colorectal cancer still has a high incidence and mortality rate, according to a report from the American Cancer Society. Colorectal cancer has a high prevalence in patients with inflammatory bowel disease. Oxidative stress, including reactive oxygen species (ROS) and lipid peroxidation, has been known to cause inflammatory diseases and malignant disorders. In particular, the nuclear factor erythroid 2-related factor 2 (Nrf2)/Kelch-like ECH-related protein 1 (KEAP1) pathway is well known to protect cells from oxidative stress and inflammation. Nrf2 was first found in the homolog of the hematopoietic transcription factor p45 NF-E2, and the transcription factor Nrf2 is a member of the Cap ‘N’ Collar family. KEAP1 is well known as a negative regulator that rapidly degrades Nrf2 through the proteasome system. A range of evidence has shown that consumption of phytochemicals has a preventive or inhibitory effect on cancer progression or proliferation, depending on the stage of colorectal cancer. Therefore, the discovery of phytochemicals regulating the Nrf2/KEAP1 axis and Citation: Lee, D.-Y.; Song, M.-Y.; verification of their efficacy have attracted scientific attention. In this review, we summarize the role Kim, E.-H. Role of Oxidative Stress of oxidative stress and the Nrf2/KEAP1 signaling pathway in colorectal cancer, and the possible and Nrf2/KEAP1 Signaling in utility of phytochemicals with respect to the regulation of the Nrf2/KEAP1 axis in colorectal cancer. -

The C718T Polymorphism in the 3″-Untranslated
Hypertension Research (2012) 35, 507–512 & 2012 The Japanese Society of Hypertension All rights reserved 0916-9636/12 www.nature.com/hr ORIGINAL ARTICLE The C718T polymorphism in the 3¢-untranslated region of glutathione peroxidase-4 gene is a predictor of cerebral stroke in patients with essential hypertension Alexey V Polonikov1, Ekaterina K Vialykh2, Mikhail I Churnosov3, Thomas Illig4, Maxim B Freidin5, Oksana V Vasil¢eva1, Olga Yu Bushueva1, Valentina N Ryzhaeva1, Irina V Bulgakova1 and Maria A Solodilova1 In the present study we have investigated the association of three single nucleotide polymorphisms in glutathione peroxidase (GPx) genes GPX1 rs1050450 (P198L), GPX3 rs2070593 (G930A) and GPX4 rs713041 (T718C) with the risk of cerebral stroke (CS) in patients with essential hypertension (EH). A total of 667 unrelated EH patients of Russian origin, including 306 hypertensives (the EH–CS group) who suffered from CS and 361 people (the EH–CS group) who did not have cerebrovascular accidents, were enrolled in the study. The variant allele 718C of the GPX4 gene was found to be significantly associated with an increased risk of CS in hypertensive patients (odds ratio (OR) 1.53, 95% confidence interval (CI) 1.23–1.90, Padj¼0.0003). The prevalence of the 718TC and 718CC genotypes of the GPX4 gene was higher in the EH–CS group than the EH-alone group (OR¼2.12, 95%CI 1.42–3.16, Padj¼0.0018). The association of the variant GPX4 genotypes with the increased risk of CS in hypertensives remained statistically significant after adjusting for confounding variables such as sex, body mass index (BMI), blood pressure and antihypertensive medication use (OR¼2.18, 95%CI 1.46–3.27, P¼0.0015). -

Programmed Cell-Death by Ferroptosis: Antioxidants As Mitigators
International Journal of Molecular Sciences Review Programmed Cell-Death by Ferroptosis: Antioxidants as Mitigators Naroa Kajarabille 1 and Gladys O. Latunde-Dada 2,* 1 Nutrition and Obesity Group, Department of Nutrition and Food Sciences, University of the Basque Country (UPV/EHU), 01006 Vitoria, Spain; [email protected] 2 King’s College London, Department of Nutritional Sciences, Faculty of Life Sciences and Medicine, Franklin-Wilkins Building, 150 Stamford Street, London SE1 9NH, UK * Correspondence: [email protected] Received: 9 September 2019; Accepted: 2 October 2019; Published: 8 October 2019 Abstract: Iron, the fourth most abundant element in the Earth’s crust, is vital in living organisms because of its diverse ligand-binding and electron-transfer properties. This ability of iron in the redox cycle as a ferrous ion enables it to react with H2O2, in the Fenton reaction, to produce a hydroxyl radical ( OH)—one of the reactive oxygen species (ROS) that cause deleterious oxidative damage • to DNA, proteins, and membrane lipids. Ferroptosis is a non-apoptotic regulated cell death that is dependent on iron and reactive oxygen species (ROS) and is characterized by lipid peroxidation. It is triggered when the endogenous antioxidant status of the cell is compromised, leading to lipid ROS accumulation that is toxic and damaging to the membrane structure. Consequently, oxidative stress and the antioxidant levels of the cells are important modulators of lipid peroxidation that induce this novel form of cell death. Remedies capable of averting iron-dependent lipid peroxidation, therefore, are lipophilic antioxidants, including vitamin E, ferrostatin-1 (Fer-1), liproxstatin-1 (Lip-1) and possibly potent bioactive polyphenols. -

Extracellular Vesicles Derived from Induced Pluripotent Stem Cells Promote Renoprotection in Acute Kidney Injury Model
cells Article Extracellular Vesicles Derived from Induced Pluripotent Stem Cells Promote Renoprotection in Acute Kidney Injury Model Federica Collino 1,2,3 , Jarlene A. Lopes 1,2,4, Marta Tapparo 5, Giovane G. Tortelote 1,6, Taís H. Kasai-Brunswick 1,2,4, Gustavo M.C. Lopes 1,2,4, Douglas B. Almeida 1,2,4, Renata Skovronova 7, Camila H. C. Wendt 1, Kildare R. de Miranda 1,4,8, Benedetta Bussolati 7 , Adalberto Vieyra 1,2,4,9,* and Rafael Soares Lindoso 1,2,4,* 1 Institute of Biophysics Carlos Chagas Filho, Federal University of Rio de Janeiro, 21941-902 Rio de Janeiro, Brazil; [email protected] (F.C.); [email protected] (J.A.L.); [email protected] (G.G.T.); [email protected] (T.H.K.-B.); [email protected] (G.M.C.L.); [email protected] (D.B.A.); [email protected] (C.H.C.W.); [email protected] (K.R.d.M.) 2 National Institute of Science and Technology for Regenerative Medicine-REGENERA, Federal University of Rio de Janeiro, 21941-902 Rio de Janeiro, Brazil 3 Department of Biomedical Sciences, University of Padova, 35131 Padua, Italy 4 National Center for Structural Biology and Bioimaging/CENABIO, Federal University of Rio de Janeiro, 21941-902 Rio de Janeiro, Brazil 5 Department of Medical Sciences, Molecular Biotechnology Center, University of Torino, 10126 Torino, Italy; [email protected] 6 Department of Pediatrics’ Section of Pediatric Nephrology, Tulane University School of Medicine, New Orleans, LA 70112, USA 7 Department of Molecular Biotechnology and Health Sciences, University of Torino, -
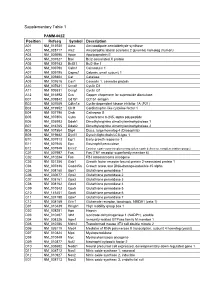
Supplementary Table 1
Supplementary Table 1 PAMM-062Z Position Refseq Symbol Description A01 NM_013930 Aass Aminoadipate-semialdehyde synthase A02 NM_028717 Als2 Amyotrophic lateral sclerosis 2 (juvenile) homolog (human) A03 NM_009696 Apoe Apolipoprotein E A04 NM_007527 Bax Bcl2-associated X protein A05 NM_009743 Bcl2l1 Bcl2-like 1 A06 NM_009790 Calm1 Calmodulin 1 A07 NM_009795 Capns1 Calpain, small subunit 1 A08 NM_009804 Cat Catalase A09 NM_007616 Cav1 Caveolin 1, caveolae protein A10 NM_007631 Ccnd1 Cyclin D1 A11 NM_009831 Ccng1 Cyclin G1 A12 NM_016892 Ccs Copper chaperone for superoxide dismutase B01 NM_009842 Cd151 CD151 antigen B02 NM_007669 Cdkn1a Cyclin-dependent kinase inhibitor 1A (P21) B03 NM_019952 Clcf1 Cardiotrophin-like cytokine factor 1 B04 NM_007798 Ctsb Cathepsin B B05 NM_007806 Cyba Cytochrome b-245, alpha polypeptide B06 NM_026993 Ddah1 Dimethylarginine dimethylaminohydrolase 1 B07 NM_016765 Ddah2 Dimethylarginine dimethylaminohydrolase 2 B08 NM_007864 Dlg4 Discs, large homolog 4 (Drosophila) B09 NM_019682 Dynll1 Dynein light chain LC8-type 1 B10 NM_007913 Egr1 Early growth response 1 B11 NM_007946 Epx Eosinophil peroxidase B12 NM_007949 Ercc2 Excision repair cross-complementing rodent repair deficiency, complementation group 2 C01 NM_007987 Fas Fas (TNF receptor superfamily member 6) C02 NM_010234 Fos FBJ osteosarcoma oncogene C03 NM_021356 Gab1 Growth factor receptor bound protein 2-associated protein 1 C04 NM_007836 Gadd45a Growth arrest and DNA-damage-inducible 45 alpha C05 NM_008160 Gpx1 Glutathione peroxidase 1 C06 NM_030677 Gpx2 Glutathione -

Catalysis of Peroxide Reduction by Fast Reacting Protein Thiols Focus Review †,‡ †,‡ ‡,§ ‡,§ ∥ Ari Zeida, Madia Trujillo, Gerardo Ferrer-Sueta, Ana Denicola, Darío A
Review Cite This: Chem. Rev. 2019, 119, 10829−10855 pubs.acs.org/CR Catalysis of Peroxide Reduction by Fast Reacting Protein Thiols Focus Review †,‡ †,‡ ‡,§ ‡,§ ∥ Ari Zeida, Madia Trujillo, Gerardo Ferrer-Sueta, Ana Denicola, Darío A. Estrin, and Rafael Radi*,†,‡ † ‡ § Departamento de Bioquímica, Centro de Investigaciones Biomedicaś (CEINBIO), Facultad de Medicina, and Laboratorio de Fisicoquímica Biologica,́ Facultad de Ciencias, Universidad de la Republica,́ 11800 Montevideo, Uruguay ∥ Departamento de Química Inorganica,́ Analítica y Química-Física and INQUIMAE-CONICET, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, 2160 Buenos Aires, Argentina ABSTRACT: Life on Earth evolved in the presence of hydrogen peroxide, and other peroxides also emerged before and with the rise of aerobic metabolism. They were considered only as toxic byproducts for many years. Nowadays, peroxides are also regarded as metabolic products that play essential physiological cellular roles. Organisms have developed efficient mechanisms to metabolize peroxides, mostly based on two kinds of redox chemistry, catalases/peroxidases that depend on the heme prosthetic group to afford peroxide reduction and thiol-based peroxidases that support their redox activities on specialized fast reacting cysteine/selenocysteine (Cys/Sec) residues. Among the last group, glutathione peroxidases (GPxs) and peroxiredoxins (Prxs) are the most widespread and abundant families, and they are the leitmotif of this review. After presenting the properties and roles of different peroxides in biology, we discuss the chemical mechanisms of peroxide reduction by low molecular weight thiols, Prxs, GPxs, and other thiol-based peroxidases. Special attention is paid to the catalytic properties of Prxs and also to the importance and comparative outlook of the properties of Sec and its role in GPxs.