Synthetic Lethal Screening in the Mammalian Central Nervous System Identifies Gpx6 As a Modulator of Huntington’S Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DNA Repair and Synthetic Lethality
Int J Oral Sci (2011) 3:176-179. www.ijos.org.cn REVIEW DNA repair and synthetic lethality Gong-she Guo1, Feng-mei Zhang1, Rui-jie Gao2, Robert Delsite4, Zhi-hui Feng1,3*, Simon N. Powell4* 1School of Public Health, Shandong University, Jinan 250012, China; 2Second People’s Hospital of Weifang, Weifang 261041, China; 3Department of Radiation Oncology, Washington University in St Louis, St Louis MO 63108, USA; 4Department of Radiation Oncology, Memorial Sloan Kettering Cancer Center, New York NY 10065, USA Tumors often have DNA repair defects, suggesting additional inhibition of other DNA repair pathways in tumors may lead to synthetic lethality. Accumulating data demonstrate that DNA repair-defective tumors, in particular homologous recombination (HR), are highly sensitive to DNA-damaging agents. Thus, HR-defective tumors exhibit potential vulnerability to the synthetic lethality approach, which may lead to new therapeutic strategies. It is well known that poly (adenosine diphosphate (ADP)-ribose) polymerase (PARP) inhibitors show the synthetically lethal effect in tumors defective in BRCA1 or BRCA2 genes encoded proteins that are required for efficient HR. In this review, we summarize the strategies of targeting DNA repair pathways and other DNA metabolic functions to cause synthetic lethality in HR-defective tumor cells. Keywords: DNA repair; homologous recombination; synthetic lethality; BRCA; Rad52 International Journal of Oral Science (2011) 3: 176-179. doi: 10.4248/IJOS11064 Introduction polymerase (PARP) inhibitors are specifically toxic to homologous recombination (HR)-defective cells [3-4]. Synthetic lethality is defined as a genetic combination Playing a key role in maintaining genetic stability, HR is of mutations in two or more genes that leads to cell a major repair pathway for double-strand breaks (DSBs) death, whereas a mutation in any one of the genes does utilizing undamaged homologous DNA sequence [5-6]. -

SUPPLEMENTARY DATA Supplementary Figure 1. The
SUPPLEMENTARY DATA Supplementary Figure 1. The results of Sirt1 activation in primary cultured TG cells using adenoviral system. GFP expression served as the control (n = 4 per group). Supplementary Figure 2. Two different Sirt1 activators, SRT1720 (0.5 µM or 1 µM ) and RSV (1µM or 10µM), induced the upregulation of Sirt1 in the primary cultured TG cells (n = 4 per group). ©2016 American Diabetes Association. Published online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-1283/-/DC1 SUPPLEMENTARY DATA Supplementary Table 1. Primers used in qPCR Gene Name Primer Sequences Product Size (bp) Sirt1 F: tgccatcatgaagccagaga 241 (NM_001159589) R: aacatcgcagtctccaagga NOX4 F: tgtgcctttattgtgcggag 172 (NM_001285833.1) R: gctgatacactggggcaatg Supplementary Table 2. Antibodies used in Western blot or Immunofluorescence Antibody Company Cat. No Isotype Dilution Sirt1 Santa Cruz * sc-15404 Rabbit IgG 1/200 NF200 Sigma** N5389 Mouse IgG 1/500 Tubulin R&D# MAB1195 Mouse IgG 1/500 NOX4 Abcam† Ab133303 Rabbit IgG 1/500 NOX2 Abcam Ab129068 Rabbit IgG 1/500 phospho-AKT CST‡ #4060 Rabbit IgG 1/500 EGFR CST #4267 Rabbit IgG 1/500 Ki67 Santa Cruz sc-7846 Goat IgG 1/500 * Santa Cruz Biotechnology, Santa Cruz, CA, USA ** Sigma aldrich, Shanghai, China # R&D Systems Inc, Minneapolis, MN, USA † Abcam, Inc., Cambridge, MA, USA ‡ Cell Signaling Technology, Inc., Danvers, MA, USA ©2016 American Diabetes Association. Published online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-1283/-/DC1 SUPPLEMENTARY DATA Supplementary -

The Tumor Therapy Landscape of Synthetic Lethality
ARTICLE https://doi.org/10.1038/s41467-021-21544-2 OPEN The tumor therapy landscape of synthetic lethality Biyu Zhang1,4, Chen Tang1,4, Yanli Yao2,4, Xiaohan Chen1, Chi Zhou 1, Zhiting Wei1, Feiyang Xing1, Lan Chen2, ✉ ✉ Xiang Cai3, Zhiyuan Zhang2, Shuyang Sun 2 & Qi Liu 1 Synthetic lethality is emerging as an important cancer therapeutic paradigm, while the comprehensive selective treatment opportunities for various tumors have not yet been explored. We develop the Synthetic Lethality Knowledge Graph (SLKG), presenting the tumor therapy landscape of synthetic lethality (SL) and synthetic dosage lethality (SDL). SLKG 1234567890():,; integrates the large-scale entity of different tumors, drugs and drug targets by exploring a comprehensive set of SL and SDL pairs. The overall therapy landscape is prioritized to identify the best repurposable drug candidates and drug combinations with literature supports, in vitro pharmacologic evidence or clinical trial records. Finally, cladribine, an FDA-approved multiple sclerosis treatment drug, is selected and identified as a repurposable drug for treating melanoma with CDKN2A mutation by in vitro validation, serving as a demonstrating SLKG utility example for novel tumor therapy discovery. Collectively, SLKG forms the com- putational basis to uncover cancer-specific susceptibilities and therapy strategies based on the principle of synthetic lethality. 1 Translational Medical Center for Stem Cell Therapy and Institute for Regenerative Medicine, Shanghai East Hospital, Bioinformatics Department, School of Life Sciences and Technology, Tongji University, Shanghai, China. 2 Department of Oral and Maxillofacial-Head Neck Oncology, Shanghai Ninth People’s Hospital, College of Stomatology, Shanghai Jiao Tong University School of Medicine, Shanghai, China. -

GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis Giovanni C
REVIEW GPX4 www.proteomics-journal.com GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis Giovanni C. Forcina and Scott J. Dixon* formation of toxic radicals (e.g., R-O•).[5] Oxygen is necessary for aerobic metabolism but can cause the harmful The eight mammalian GPX proteins fall oxidation of lipids and other macromolecules. Oxidation of cholesterol and into three clades based on amino acid phospholipids containing polyunsaturated fatty acyl chains can lead to lipid sequence similarity: GPX1 and GPX2; peroxidation, membrane damage, and cell death. Lipid hydroperoxides are key GPX3, GPX5, and GPX6; and GPX4, GPX7, and GPX8.[6] GPX1–4 and 6 (in intermediates in the process of lipid peroxidation. The lipid hydroperoxidase humans) are selenoproteins that contain glutathione peroxidase 4 (GPX4) converts lipid hydroperoxides to lipid an essential selenocysteine in the enzyme + alcohols, and this process prevents the iron (Fe2 )-dependent formation of active site, while GPX5, 6 (in mouse and toxic lipid reactive oxygen species (ROS). Inhibition of GPX4 function leads to rats), 7, and 8 use an active site cysteine lipid peroxidation and can result in the induction of ferroptosis, an instead. Unlike other family members, GPX4 (PHGPx) can act as a phospholipid iron-dependent, non-apoptotic form of cell death. This review describes the hydroperoxidase to reduce lipid perox- formation of reactive lipid species, the function of GPX4 in preventing ides to lipid alcohols.[7,8] Thus,GPX4ac- oxidative lipid damage, and the link between GPX4 dysfunction, lipid tivity is essential to maintain lipid home- oxidation, and the induction of ferroptosis. ostasis in the cell, prevent the accumula- tion of toxic lipid ROS and thereby block the onset of an oxidative, iron-dependent, non-apoptotic mode of cell death termed 1. -

Extracellular Vesicles Derived from Induced Pluripotent Stem Cells Promote Renoprotection in Acute Kidney Injury Model
cells Article Extracellular Vesicles Derived from Induced Pluripotent Stem Cells Promote Renoprotection in Acute Kidney Injury Model Federica Collino 1,2,3 , Jarlene A. Lopes 1,2,4, Marta Tapparo 5, Giovane G. Tortelote 1,6, Taís H. Kasai-Brunswick 1,2,4, Gustavo M.C. Lopes 1,2,4, Douglas B. Almeida 1,2,4, Renata Skovronova 7, Camila H. C. Wendt 1, Kildare R. de Miranda 1,4,8, Benedetta Bussolati 7 , Adalberto Vieyra 1,2,4,9,* and Rafael Soares Lindoso 1,2,4,* 1 Institute of Biophysics Carlos Chagas Filho, Federal University of Rio de Janeiro, 21941-902 Rio de Janeiro, Brazil; [email protected] (F.C.); [email protected] (J.A.L.); [email protected] (G.G.T.); [email protected] (T.H.K.-B.); [email protected] (G.M.C.L.); [email protected] (D.B.A.); [email protected] (C.H.C.W.); [email protected] (K.R.d.M.) 2 National Institute of Science and Technology for Regenerative Medicine-REGENERA, Federal University of Rio de Janeiro, 21941-902 Rio de Janeiro, Brazil 3 Department of Biomedical Sciences, University of Padova, 35131 Padua, Italy 4 National Center for Structural Biology and Bioimaging/CENABIO, Federal University of Rio de Janeiro, 21941-902 Rio de Janeiro, Brazil 5 Department of Medical Sciences, Molecular Biotechnology Center, University of Torino, 10126 Torino, Italy; [email protected] 6 Department of Pediatrics’ Section of Pediatric Nephrology, Tulane University School of Medicine, New Orleans, LA 70112, USA 7 Department of Molecular Biotechnology and Health Sciences, University of Torino, -
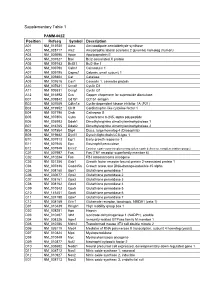
Supplementary Table 1
Supplementary Table 1 PAMM-062Z Position Refseq Symbol Description A01 NM_013930 Aass Aminoadipate-semialdehyde synthase A02 NM_028717 Als2 Amyotrophic lateral sclerosis 2 (juvenile) homolog (human) A03 NM_009696 Apoe Apolipoprotein E A04 NM_007527 Bax Bcl2-associated X protein A05 NM_009743 Bcl2l1 Bcl2-like 1 A06 NM_009790 Calm1 Calmodulin 1 A07 NM_009795 Capns1 Calpain, small subunit 1 A08 NM_009804 Cat Catalase A09 NM_007616 Cav1 Caveolin 1, caveolae protein A10 NM_007631 Ccnd1 Cyclin D1 A11 NM_009831 Ccng1 Cyclin G1 A12 NM_016892 Ccs Copper chaperone for superoxide dismutase B01 NM_009842 Cd151 CD151 antigen B02 NM_007669 Cdkn1a Cyclin-dependent kinase inhibitor 1A (P21) B03 NM_019952 Clcf1 Cardiotrophin-like cytokine factor 1 B04 NM_007798 Ctsb Cathepsin B B05 NM_007806 Cyba Cytochrome b-245, alpha polypeptide B06 NM_026993 Ddah1 Dimethylarginine dimethylaminohydrolase 1 B07 NM_016765 Ddah2 Dimethylarginine dimethylaminohydrolase 2 B08 NM_007864 Dlg4 Discs, large homolog 4 (Drosophila) B09 NM_019682 Dynll1 Dynein light chain LC8-type 1 B10 NM_007913 Egr1 Early growth response 1 B11 NM_007946 Epx Eosinophil peroxidase B12 NM_007949 Ercc2 Excision repair cross-complementing rodent repair deficiency, complementation group 2 C01 NM_007987 Fas Fas (TNF receptor superfamily member 6) C02 NM_010234 Fos FBJ osteosarcoma oncogene C03 NM_021356 Gab1 Growth factor receptor bound protein 2-associated protein 1 C04 NM_007836 Gadd45a Growth arrest and DNA-damage-inducible 45 alpha C05 NM_008160 Gpx1 Glutathione peroxidase 1 C06 NM_030677 Gpx2 Glutathione -

Catalysis of Peroxide Reduction by Fast Reacting Protein Thiols Focus Review †,‡ †,‡ ‡,§ ‡,§ ∥ Ari Zeida, Madia Trujillo, Gerardo Ferrer-Sueta, Ana Denicola, Darío A
Review Cite This: Chem. Rev. 2019, 119, 10829−10855 pubs.acs.org/CR Catalysis of Peroxide Reduction by Fast Reacting Protein Thiols Focus Review †,‡ †,‡ ‡,§ ‡,§ ∥ Ari Zeida, Madia Trujillo, Gerardo Ferrer-Sueta, Ana Denicola, Darío A. Estrin, and Rafael Radi*,†,‡ † ‡ § Departamento de Bioquímica, Centro de Investigaciones Biomedicaś (CEINBIO), Facultad de Medicina, and Laboratorio de Fisicoquímica Biologica,́ Facultad de Ciencias, Universidad de la Republica,́ 11800 Montevideo, Uruguay ∥ Departamento de Química Inorganica,́ Analítica y Química-Física and INQUIMAE-CONICET, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, 2160 Buenos Aires, Argentina ABSTRACT: Life on Earth evolved in the presence of hydrogen peroxide, and other peroxides also emerged before and with the rise of aerobic metabolism. They were considered only as toxic byproducts for many years. Nowadays, peroxides are also regarded as metabolic products that play essential physiological cellular roles. Organisms have developed efficient mechanisms to metabolize peroxides, mostly based on two kinds of redox chemistry, catalases/peroxidases that depend on the heme prosthetic group to afford peroxide reduction and thiol-based peroxidases that support their redox activities on specialized fast reacting cysteine/selenocysteine (Cys/Sec) residues. Among the last group, glutathione peroxidases (GPxs) and peroxiredoxins (Prxs) are the most widespread and abundant families, and they are the leitmotif of this review. After presenting the properties and roles of different peroxides in biology, we discuss the chemical mechanisms of peroxide reduction by low molecular weight thiols, Prxs, GPxs, and other thiol-based peroxidases. Special attention is paid to the catalytic properties of Prxs and also to the importance and comparative outlook of the properties of Sec and its role in GPxs. -

Glutathione Peroxidase 3 (GPX3) (102-114) Goat Polyclonal Antibody Product Data
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for AP22523PU-N Glutathione Peroxidase 3 (GPX3) (102-114) Goat Polyclonal Antibody Product data: Product Type: Primary Antibodies Applications: ELISA, IHC, WB Recommended Dilution: ELISA: 1/1000. Immunohistochemistry on Paraffin Sections: 5 µg/ml. Western Blot: 1 - 3 µg/ml. Reactivity: Bat, Bovine, Canine, Equine, Human, Monkey, Mouse, Porcine, Rat Host: Goat Clonality: Polyclonal Immunogen: Synthetic peptide from internal region of human GPX3 Specificity: This antibody detects Glutathione peroxidase 3 at aa 102-114. It may cross-react with epididymis-specific GPX5 and olfactory epithelium-speciific GPX6. Formulation: Tris saline buffer, pH 7.3, 0.5% BSA, 0.02% sodium azide State: Liquid Ig fraction Concentration: lot specific Purification: Immunoaffinity chromatography Conjugation: Unconjugated Storage: Store at 2 - 8 °C for up to three months or (in aliquots) at -20 °C for longer. Avoid repeated freezing and thawing. Stability: Shelf life: one year from despatch. Gene Name: Homo sapiens glutathione peroxidase 3 (GPX3), transcript variant 1 Database Link: Entrez Gene 14778 MouseEntrez Gene 64317 RatEntrez Gene 2878 Human P22352 Background: Glutathione peroxidase catalyzes the reduction of hydrogen peroxide, organic hydroperoxide, and lipid peroxides by reduced glutathione and functions in the protection of cells against oxidative damage. Human plasma glutathione peroxidase has been shown to be a selenium- containing enzyme. GPX3 expression appears to be tissue-specific. Synonyms: GPX-3, GPXP, GSHPx-3, GPx-3, GSHPx-P, GSHPXP, GPx-P, GPXP Protein Families: Druggable Genome, Secreted Protein This product is to be used for laboratory only. -

The Emerging Roles of Antioxidant Enzymes by Dietary Phytochemicals in Vascular Diseases
life Review The Emerging Roles of Antioxidant Enzymes by Dietary Phytochemicals in Vascular Diseases Seung Eun Lee and Yong Seek Park * Department of Microbiology, School of Medicine, Kyung Hee University, Seoul 02447, Korea; [email protected] * Correspondence: [email protected]; Tel.: +82-02-961-0267 Abstract: Vascular diseases are major causes of death worldwide, causing pathologies including diabetes, atherosclerosis, and chronic obstructive pulmonary disease (COPD). Exposure of the vascular system to a variety of stressors and inducers has been implicated in the development of various human diseases, including chronic inflammatory diseases. In the vascular wall, antioxidant enzymes form the first line of defense against oxidative stress. Recently, extensive research into the beneficial effects of phytochemicals has been conducted; phytochemicals are found in commonly used spices, fruits, and herbs, and are used to prevent various pathologic conditions, including vascular diseases. The present review aims to highlight the effects of dietary phytochemicals role on antioxidant enzymes in vascular diseases. Keywords: vascular diseases; antioxidant enzymes; bioactive compounds 1. Introduction Citation: Lee, S.E.; Park, Y.S. The Vascular diseases are responsible for numerous deaths annually worldwide. The Emerging Roles of Antioxidant pathogenesis of vascular disease involves the activation of pro-inflammatory signaling Enzymes by Dietary Phytochemicals pathways, expression of cytokines/chemokines, and elevated oxidative stress. Exposure in Vascular Diseases. Life 2021, 11, 199. https://doi.org/10.3390/ to oxidative stress may directly injure the vasculature and induce vascular dysfunction life11030199 by producing dysregulation of the immune response. Oxidative stress is caused by an imbalance between the production and accumulation of reactive oxygen species (ROS) and Academic Editor: Payaningal the capacity of antioxidant defense mechanisms favoring oxidants [1]. -

Characterization of Cytosolic Glutathione Peroxidase And
Aquatic Toxicology 130–131 (2013) 97–111 Contents lists available at SciVerse ScienceDirect Aquatic Toxicology jou rnal homepage: www.elsevier.com/locate/aquatox Characterization of cytosolic glutathione peroxidase and phospholipid-hydroperoxide glutathione peroxidase genes in rainbow trout (Oncorhynchus mykiss) and their modulation by in vitro selenium exposure a a b a d c a,∗ D. Pacitti , T. Wang , M.M. Page , S.A.M. Martin , J. Sweetman , J. Feldmann , C.J. Secombes a Scottish Fish Immunology Research Centre, Institute of Biological and Environmental Sciences, University of Aberdeen, Aberdeen AB24 2TZ, United Kingdom b Integrative and Environmental Physiology, Institute of Biological and Environmental Sciences, University of Aberdeen, Aberdeen AB24 2TZ, United Kingdom c Trace Element Speciation Laboratory, Department of Chemistry, University of Aberdeen, Aberdeen AB24 3UE, United Kingdom d Alltech Biosciences Centre, Sarney, Summerhill Rd, Dunboyne, Country Meath, Ireland a r t i c l e i n f o a b s t r a c t Article history: Selenium (Se) is an oligonutrient with both essential biological functions and recognized harmful effects. Received 4 July 2012 As the selenocysteine (SeCys) amino acid, selenium is integrated in several Se-containing proteins Received in revised form (selenoproteins), many of which are fundamental for cell homeostasis. Nevertheless, selenium may exert 19 December 2012 toxic effects at levels marginally above those required, mainly through the generation of reactive oxygen Accepted 20 December 2012 species (ROS). The selenium chemical speciation can strongly affect the bioavailability of this metal and its impact on metabolism, dictating the levels that can be beneficial or detrimental towards an organism. -

Involvement of Glutathione Peroxidases in the Occurrence And
Zhang et al. J Transl Med (2020) 18:247 https://doi.org/10.1186/s12967-020-02420-x Journal of Translational Medicine REVIEW Open Access Involvement of glutathione peroxidases in the occurrence and development of breast cancers Man‑Li Zhang1†, Hua‑Tao Wu2†, Wen‑Jia Chen1,3, Ya Xu1, Qian‑Qian Ye1,3, Jia‑Xin Shen4 and Jing Liu1,3* Abstract Glutathione peroxidases (GPxs) belong to a family of enzymes that is important in organisms; these enzymes pro‑ mote hydrogen peroxide metabolism and protect cell membrane structure and function from oxidative damage. Based on the establishment and development of the theory of the pathological roles of free radicals, the role of GPxs has gradually attracted researchers’ attention, and the involvement of GPxs in the occurrence and development of malignant tumors has been shown. On the other hand, the incidence of breast cancer in increasing, and breast cancer has become the leading cause of cancer‑related death in females worldwide; breast cancer is thought to be related to the increased production of reactive oxygen species, indicating the involvement of GPxs in these processes. Therefore, this article focused on the molecular mechanism and function of GPxs in the occurrence and development of breast cancer to understand their role in breast cancer and to provide a new theoretical basis for the treatment of breast cancer. Keywords: Glutathione peroxidase, Breast cancer, Reactive oxygen species, Occurrence Background [4]. However, the mechanisms of the occurrence, devel- Breast cancer has become the most common cancer and opment, and metastasis of breast cancer are very com- the leading cause of cancer-related deaths in females plicated and overlap, suggesting the necessity of diferent worldwide, according to a status report on the global therapies to treat diferent subtypes of breast cancer. -

Expression Silencing of Glutathione Peroxidase 4 in Mouse Erythroleukemia Cells Delays in Vitro Erythropoiesis
International Journal of Molecular Sciences Article Expression Silencing of Glutathione Peroxidase 4 in Mouse Erythroleukemia Cells Delays In Vitro Erythropoiesis Marlena Rademacher, Hartmut Kuhn and Astrid Borchert * Charité—Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Institute of Biochemistry, Charitéplatz 1, 10117 Berlin, Germany; [email protected] (M.R.); [email protected] (H.K.) * Correspondence: [email protected]; Tel.: +49-30-450-528-034 Abstract: Among the eight human glutathione peroxidase isoforms, glutathione peroxidase 4 (GPX4) is the only enzyme capable of reducing complex lipid peroxides to the corresponding alcohols. In mice, corruption of the Gpx4 gene leads to embryonic lethality and more detailed expression silencing studies have implicated the enzyme in several physiological processes (e.g., embryonal cerebroge- nesis, neuronal function, male fertility). Experiments with conditional knockout mice, in which expression of the Gpx4 gene was silenced in erythroid precursors, indicated a role of Gpx4 in erythro- poiesis. To test this hypothesis in a cellular in vitro model we transfected mouse erythroleukemia cells with a Gpx4 siRNA construct and followed the expression kinetics of erythropoietic gene products. Our data indicate that Gpx4 is expressed at high levels in mouse erythroleukemia cells and that expression silencing of the Gpx4 gene delays in vitro erythropoiesis. However, heterozygous expression of a catalytically inactive Gpx4 mutant (Gpx4+/Sec46Ala) did not induce a defective ery- thropoietic phenotype in different in vivo and ex vivo models. These data suggest that Gpx4 plays a role in erythroid differentiation of mouse erythroleukemia cells but that heterozygous expression of a Citation: Rademacher, M.; Kuhn, H.; catalytically inactive Gpx4 is not sufficient to compromise in vivo and ex vivo erythropoiesis.