Rodent Somatosensory Thalamocortical Circuitry: Neurons, Synapses, and Connectivity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download The
ACTIONS OF ISOVALINE AND ENDOGENOUS AMINO ACIDS ON INHIBITORY RECEPTORS IN VENTROBASAL THALAMUS by JAMES EDWARD COOKE BSc, Carleton University, 2002 MSc, Carleton University, 2004 A THESIS SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in THE FACULTY OF GRADUATE STUDIES (Pharmacology) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) August, 2010 © James Edward Cooke, 2010 ABSTRACT This thesis consists of three manuscripts that examine the effects of amino acids and inhibitory neurotransmission in ventrobasal thalamus, a region of the brain responsible for processing nociceptive information. In the first manuscript we examined the possibility that a proposed antagonist of receptors for endogenous amino acids was selective for -amino acids. In the second manuscript, we determined the ionic mechanism of action of isovaline, a non-biogenic amino acid with chemical similarity to glycine and GABA. In the third manuscript, we determined that the inhibitory action of isovaline is mediated by metabotropic receptors, likely GABAB. In the first manuscript we used whole-cell patch clamp electrophysiology and immunohistochemistry to examine the differential antagonism of GABAAergic IPSCs by a proposed -amino acid antagonist, TAG. In IPSCs that were attributable to both GABAergic and glycinergic stimulation, TAG significantly reduced both components. TAG had no effect in purely GABAAergic IPSCs. Our data supports the hypothesis that a specific GABAA subunit, 4, is sensitive to the -amino acid antagonist, TAG. The second manuscript examines the ionic mechanism of action of isovaline, demonstrated to have analgesic properties in animal models. Isovaline inhibited action potential firing of thalamocortical neurons by activating a long-lasting potassium conductance that was insensitive to the glycine antagonist, strychnine. -

Cortex and Thalamus Lecture.Pptx
Cerebral Cortex and Thalamus Hyperbrain Ch 2 Monica Vetter, PhD January 24, 2013 Learning Objectives: • Anatomy of the lobes of the cortex • Relationship of thalamus to cortex • Layers and connectivity of the cortex • Vascular supply to cortex • Understand the location and function of hypothalamus and pituitary • Anatomy of the basal ganglia • Primary functions of the different lobes/ cortical regions – neurological findings 1 Types of Cortex • Sensory (Primary) • Motor (Primary) • Unimodal association • Multimodal association - necessary for language, reason, plan, imagine, create Note: • Gyri • Sulci • Fissures • Lobes 2 The Thalamus is highly interconnected with the cerebral cortex, and handles most information traveling to or from the cortex. “Specific thalamic Ignore nuclei” – have well- names of defined sensory or thalamic nuclei for motor functions now - A few Other nuclei have will more distributed reappear later function 3 Thalamus Midbrain Pons Limbic lobe = cingulate gyrus Structure of Neocortex (6 layers) white matter gray matter Pyramidal cells 4 Connectivity of neurons in different cortical layers Afferents = inputs Efferents = outputs (reciprocal) brainstem etc Eg. Motor – Eg. Sensory – more efferent more afferent output input Cortico- cortical From Thalamus To spinal cord, brainstem etc. To Thalamus Afferent and efferent connections to different ….Depending on whether they have more layers of cortex afferent or efferent connections 5 Different areas of cortex were defined by differences in layer thickness, and size and -

The Human Thalamus Is an Integrative Hub for Functional Brain Networks
5594 • The Journal of Neuroscience, June 7, 2017 • 37(23):5594–5607 Behavioral/Cognitive The Human Thalamus Is an Integrative Hub for Functional Brain Networks X Kai Hwang, Maxwell A. Bertolero, XWilliam B. Liu, and XMark D’Esposito Helen Wills Neuroscience Institute and Department of Psychology, University of California, Berkeley, Berkeley, California 94720 The thalamus is globally connected with distributed cortical regions, yet the functional significance of this extensive thalamocortical connectivityremainslargelyunknown.Byperforminggraph-theoreticanalysesonthalamocorticalfunctionalconnectivitydatacollected from human participants, we found that most thalamic subdivisions display network properties that are capable of integrating multi- modal information across diverse cortical functional networks. From a meta-analysis of a large dataset of functional brain-imaging experiments, we further found that the thalamus is involved in multiple cognitive functions. Finally, we found that focal thalamic lesions in humans have widespread distal effects, disrupting the modular organization of cortical functional networks. This converging evidence suggests that the human thalamus is a critical hub region that could integrate diverse information being processed throughout the cerebral cortex as well as maintain the modular structure of cortical functional networks. Key words: brain networks; diaschisis; functional connectivity; graph theory; thalamus Significance Statement The thalamus is traditionally viewed as a passive relay station of information from sensory organs or subcortical structures to the cortex. However, the thalamus has extensive connections with the entire cerebral cortex, which can also serve to integrate infor- mation processing between cortical regions. In this study, we demonstrate that multiple thalamic subdivisions display network properties that are capable of integrating information across multiple functional brain networks. Moreover, the thalamus is engaged by tasks requiring multiple cognitive functions. -

Basal Ganglia Anatomy, Physiology, and Function Ns201c
Basal Ganglia Anatomy, Physiology, and Function NS201c Human Basal Ganglia Anatomy Basal Ganglia Circuits: The ‘Classical’ Model of Direct and Indirect Pathway Function Motor Cortex Premotor Cortex + Glutamate Striatum GPe GPi/SNr Dopamine + - GABA - Motor Thalamus SNc STN Analagous rodent basal ganglia nuclei Gross anatomy of the striatum: gateway to the basal ganglia rodent Dorsomedial striatum: -Inputs predominantly from mPFC, thalamus, VTA Dorsolateral striatum: -Inputs from sensorimotor cortex, thalamus, SNc Ventral striatum: Striatal subregions: Dorsomedial (caudate) -Inputs from vPFC, hippocampus, amygdala, Dorsolateral (putamen) thalamus, VTA Ventral (nucleus accumbens) Gross anatomy of the striatum: patch and matrix compartments Patch/Striosome: -substance P -mu-opioid receptor Matrix: -ChAT and AChE -somatostatin Microanatomy of the striatum: cell types Projection neurons: MSN: medium spiny neuron (GABA) •striatonigral projecting – ‘direct pathway’ •striatopallidal projecting – ‘indirect pathway’ Interneurons: FS: fast-spiking interneuron (GABA) LTS: low-threshold spiking interneuron (GABA) LA: large aspiny neuron (ACh) 30 um Cellular properties of striatal neurons Microanatomy of the striatum: striatal microcircuits • Feedforward inhibition (mediated by fast-spiking interneurons) • Lateral feedback inhibition (mediated by MSN collaterals) Basal Ganglia Circuits: The ‘Classical’ Model of Direct and Indirect Pathway Function Motor Cortex Premotor Cortex + Glutamate Striatum GPe GPi/SNr Dopamine + - GABA - Motor Thalamus SNc STN The simplified ‘classical’ model of basal ganglia circuit function • Information encoded as firing rate • Basal ganglia circuit is linear and unidirectional • Dopamine exerts opposing effects on direct and indirect pathway MSNs Basal ganglia motor circuit: direct pathway Motor Cortex Premotor Cortex Glutamate Striatum GPe GPi/SNr Dopamine + GABA Motor Thalamus SNc STN Direct pathway MSNs express: D1, M4 receptors, Sub. -

The Thalamus: Gateway to the Mind Lawrence M
Overview The thalamus: gateway to the mind Lawrence M. Ward∗ The thalamus of the brain is far more than the simple sensory relay it was long thought to be. From its location at the top of the brain stem it interacts directly with nearly every part of the brain. Its dense loops into and out of cortex render it functionally a seventh cortical layer. Moreover, it receives and sends connections to most subcortical areas as well. Of course it does function as a very sophisticated sensory relay and thus is of vital importance to perception. But also it functions critically in all mental operations, including attention, memory, and consciousness, likely in different ways for different processes, as indicated by the consequences of damage to its various nuclei as well as by invasive studies in nonhuman animals. It plays a critical role also in the arousal system of the brain, in emotion, in movement, and in coordinating cortical computations. Given these important functional roles, and the dearth of knowledge about the details of its nonsensory nuclei, it is an attractive target for intensive study in the future, particularly in regard to its role in healthy and impaired cognitive functioning. © 2013 John Wiley & Sons, Ltd. How to cite this article: WIREs Cogn Sci 2013. doi: 10.1002/wcs.1256 INTRODUCTION and other animals can do quite well without major chunks of cortex. Indeed, decorticate rats behave very pen nearly any textbook of neuroscience or similarly to normal rats in many ways,2 whereas sensation and perception and you will find the O de-thalamate rats die. -
Lecture 12 Notes
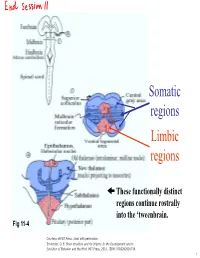
Somatic regions Limbic regions These functionally distinct regions continue rostrally into the ‘tweenbrain. Fig 11-4 Courtesy of MIT Press. Used with permission. Schneider, G. E. Brain structure and its Origins: In the Development and in Evolution of Behavior and the Mind. MIT Press, 2014. ISBN: 9780262026734. 1 Chapter 11, questions about the somatic regions: 4) There are motor neurons located in the midbrain. What movements do those motor neurons control? (These direct outputs of the midbrain are not a subject of much discussion in the chapter.) 5) At the base of the midbrain (ventral side) one finds a fiber bundle that shows great differences in relative size in different species. Give examples. What are the fibers called and where do they originate? 8) A decussating group of axons called the brachium conjunctivum also varies greatly in size in different species. It is largest in species with the largest neocortex but does not come from the neocortex. From which structure does it come? Where does it terminate? (Try to guess before you look it up.) 2 Motor neurons of the midbrain that control somatic muscles: the oculomotor nuclei of cranial nerves III and IV. At this level, the oculomotor nucleus of nerve III is present. Fibers from retina to Superior Colliculus Brachium of Inferior Colliculus (auditory pathway to thalamus, also to SC) Oculomotor nucleus Spinothalamic tract (somatosensory; some fibers terminate in SC) Medial lemniscus Cerebral peduncle: contains Red corticospinal + corticopontine fibers, + cortex to hindbrain fibers nucleus (n. ruber) Tectospinal tract Rubrospinal tract Courtesy of MIT Press. Used with permission. Schneider, G. -

Neuromodulation in Eating Disorders and Obesity: a Promising Way of Treatment?
Journal name: Neuropsychiatric Disease and Treatment Article Designation: Review Year: 2018 Volume: 14 Neuropsychiatric Disease and Treatment Dovepress Running head verso: Jáuregui-Lobera and Martínez-Quiñones Running head recto: Neuromodulation in eating disorders and obesity open access to scientific and medical research DOI: 180231 Open Access Full Text Article REVIEW Neuromodulation in eating disorders and obesity: a promising way of treatment? Ignacio Jáuregui-Lobera1 Abstract: Neuromodulation can affect the functioning of the central nervous system (CNS), José V Martínez-Quiñones2 and emotional/eating behavior is an exciting facet of that functioning. Therefore, it would be possible to offer an alternative (or complement) treatment to psychotropic medications and 1Department of Molecular Biology and Biochemical Engineering, different psychological and nutritional approaches to both eating disorders (EDs) and obe- University of Pablo de Olavide of sity. Although there are a number of publications in these areas, a systematic review has not Seville, Seville, Spain; 2Department of Neurosurgery, Mutua de been conducted to date. Abstracts, letters, conference reports, dissertations, and reviews were Accidentes de Zaragoza (Servicio de excluded. Clinical trials and controlled human clinical trials were filtered and included in this Neurocirugía), Zaragoza, Spain study. Articles included were based on the population suffering from anorexia nervosa, bulimia nervosa, binge ED, overweight, and obesity. No restrictions were placed on the sample size. Only trials investigating the effect of neuromodulation by means of deep brain stimulation (DBS), transcranial direct current stimulation (tDCS), and transcranial magnetic stimulation For personal use only. (TMS) were included. The following databases were used to conduct the search: MEDLINE/ PubMed, PsycINFO, PsycArticles, and Cochrane (Search Trials, CENTRAL). -

Dorsal “Thalamus”
Dorsal “Thalamus” Medical Neuroscience Dr. Wiegand The Diencephalon The Diencephalon InterthalamicInterthalamic adhesionadhesion ThalamusThalamus EpithalamusEpithalamus HypothalamusHypothalamus (Pineal(Pineal && Habenula)Habenula) PituitaryPituitary SubthalamusSubthalamus 1 The “Dorsal” Thalamus | Sensory integration nucleus – gateway to the cerebral cortex | Afferents from both rostral and caudal central nervous system structures | Efferents primarily to cerebral cortex via four principal “radiations” | Associated with motor, sensory, limbic and vegetative functions External medullary lamina Anterior n. 3rd Internal capsule Ventricle Medial n. Medial Lateral n. Internal capsule * Reticular n. Internal * Interthalamic adhesion medullary lamina 2 General Organization medialmedial nucleinuclei anterioranterior nuclei nuclei internalinternal medullarymedullary laminalamina laterallateral nuclei nuclei dorsaldorsal tiertier pulvinarpulvinar geniculategeniculate ventralventral tiertier bodiesbodies Frontal Section intralaminarintralaminar nucleinuclei reticularreticular nuclei nuclei 3rd Ventricle externalexternalexternalexternal medullarymedullary laminalamina internalinternal laminalamina medullarymedullary laminalamina 3 Thalamic Nuclei | Anterior | Lateral z Dorsal Tier • lateral dorsal • lateral posterior • pulvinar z Ventral Tier • ventral anterior • ventral lateral • ventral posterior (VLP & VPM) • posterior nucleus Thalamic Nuclei | Medial z medial/medial dorsal z midline nuclei | Pulvinar | Geniculate bodies | Reticular | Intralaminar -

Neuroanatomy
Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Neuroanatomy W. Jeffrey Wilson Fall 2012 \Without education we are in a horrible and deadly danger of taking educated people seriously." { Gilbert Keith Chesterton [LATEX in use { a Microsoft- & PowerPoint-free presentation] Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Blood-Brain Barrier Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Peripheral Nervous System • Somatic N.S.: skeletal muscles, skin, joints • Autonomic N.S.: internal organs, glands • Sympathetic N.S.: rapid expenditure of energy • Parasympathetic N.S.: restoration of energy Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Spinal Cord Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Brain | Ventricles Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Brain Midline Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Brain Midline Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Hindbrain Myelencephalon & Metencephalon Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Reticular -

05 Trigeminal System 2013.Pdf
Dental Neuroanatomy Thursday February 7th, 2013 David A. Morton, Ph.D. 5. THE TRIGEMINAL SYSTEM Somatic Sensation of the Face and Head Objectives 1. Outline the two pathways for facial sensation from the head. 2. Contrast facial sensation from the head and somatic sensation from the body. In what ways are they similar? Different? Try drawing this on the Haines atlas diagram at the end of the lecture. 3. Diagram the corneal reflex: the afferent and efferent limbs as well as nuclei involved in the brainstem. 4. If a person does not blink, how would you determine if the problem were in the sensory (afferent) limb, motor (efferent) limb, or brainstem interconnections for the corneal reflex? 5. Explain how a single, small medullary vascular lesion could abolish pain and temperature from the face on the right side and pain and temperature from the body on the left side. What vessel is most likely occluded? Introduction – The trigeminal system for the face and oral cavity is organized in a manner similar to the spinal cord. It has the equivalent of both the DCML pathway and the ALS pathway. The two trigeminal pathways will converge in the thalamus. The most confusing thing is that one of them descends before crossing and the other crosses immediately. Peripheral Receptors and Sensation Structures served by trigeminal system. 1. Cornea 2. Mucocutaneous tissues around mouth and nostrils. 3. Oral and nasal mucosae 4. Paranasal sinuses 5. Tongue (anterior two thirds) 6. Teeth and gums 7. Dura of anterior and middle cranial fossae 8. Skin of face to the vertex except angle of jaw 9. -

BRAIN DISORDERS “Forebrain” Disorders
The Animal Neurology & Imaging Center 5 Camino Karsten Algodones, New Mexico 87001 Phone: 505- Fax: 505- Email: [email protected] Website: www.theanic.com BRAIN DISORDERS Seizures, circling, a head turn or tilt, a change in personality and/or level of awareness, sensation deficits, visual problems, weakness and in coordination are the most common clinical signs resulting from problems affecting your pets brain. Before we can determine the exact cause of the signs (ie the diagnosis), we must perform a neurological exam in order to establish what is referred to as the “neurological localization”. On the basis of the neurological examination your dog or cat brain disorder may “localize” to one of three major areas: the “forebrain” comprised of the cerebrum and thalamus, the “brainstem” comprised of the midbrain, ponds, and Medulla, and the cerebellum Based on where the problem localizes in the brain, a list of diseases (the so-called "differential diagnosis") is then generated which includes the most likely disease conditions that could be affecting your pet’s brain. The “differential diagnoses” for any given brain problem can be narrowed down based on some specific information: the patient’s age and breed, whether the condition has come on suddenly or slowly, whether the condition is progressing or static, where the problem localizes. Below, the three primary brain localizations are considered individually because specific diseases will affect each region. “Forebrain” Disorders The most common clinical signs seen in pets with forebrain problems include seizures, visual problems, facial sensation abnormalities, circling, and changes in personality or level of consciousness. And mature dogs, epilepsy (seizures due to spontaneous, chaotic electrical activity in 2 the brain), autoimmune inflammation, tumors and strokes are the most common conditions affecting the forebrain. -

Neuromodulation Symposium
MINNESOTA NEUROMODULATION SYMPOSIUM APRIL 14 - 15, 2016 THE COMMONS HOTEL MINNEAPOLIS, MN neuromodulation.umn.edu Dear Colleagues, Welcome to the 4th Annual Minnesota Neuromodulation Symposium. Neuromodulation is a rapidly-growing field, encompassing a wide spectrum of implantable and non-invasive technology-based approaches for the treatment of neurological and psychiatric disorders. It represents an important research nexus of basic and clinical neu- roscience, and of engineering sciences. It also represents an important industrial sector in the medical device industry with products already benefiting a number of patients. A collaborative effort by all stakeholders will be needed to further expedite the process of translating basic research discoveries into applied and clinical research, industrial research and development, and even manufacturing, which will result in a more immediate impact to healthcare. This symposium is aimed at bringing together basic scientists, engineers, clinicians, industrial practitioners, entrepreneurs, and policy makers to discuss challenges and opportunities in neuromodulation and neurotechnology. Advancing the field of neuromodulation represents challenges to developing engineering methodologies, understanding mechanisms of neuromodulation at cellular and system levels, translating research to treat patients, and closely integrating the regulatory process within the research and clinical environment. We are very pleased to address these challenges through our outstanding line-up of invited speakers that represent thought leaders from academia, industry, and government, whom over the next two days will review significant progress in neuromodulation and neurotechnology, providing a glimpse into the future of this fascinating field. We are also very pleased that the symposium has attracted a record number of scientific contributions from many institutions to be presented in the Poster Session.