VSEPR Theory
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Crystal Structures
Crystal Structures Academic Resource Center Crystallinity: Repeating or periodic array over large atomic distances. 3-D pattern in which each atom is bonded to its nearest neighbors Crystal structure: the manner in which atoms, ions, or molecules are spatially arranged. Unit cell: small repeating entity of the atomic structure. The basic building block of the crystal structure. It defines the entire crystal structure with the atom positions within. Lattice: 3D array of points coinciding with atom positions (center of spheres) Metallic Crystal Structures FCC (face centered cubic): Atoms are arranged at the corners and center of each cube face of the cell. FCC continued Close packed Plane: On each face of the cube Atoms are assumed to touch along face diagonals. 4 atoms in one unit cell. a 2R 2 BCC: Body Centered Cubic • Atoms are arranged at the corners of the cube with another atom at the cube center. BCC continued • Close Packed Plane cuts the unit cube in half diagonally • 2 atoms in one unit cell 4R a 3 Hexagonal Close Packed (HCP) • Cell of an HCP lattice is visualized as a top and bottom plane of 7 atoms, forming a regular hexagon around a central atom. In between these planes is a half- hexagon of 3 atoms. • There are two lattice parameters in HCP, a and c, representing the basal and height parameters Volume respectively. 6 atoms per unit cell Coordination number – the number of nearest neighbor atoms or ions surrounding an atom or ion. For FCC and HCP systems, the coordination number is 12. For BCC it’s 8. -

Crystal Structure of a Material Is Way in Which Atoms, Ions, Molecules Are Spatially Arranged in 3-D Space
Crystalline Structures – The Basics •Crystal structure of a material is way in which atoms, ions, molecules are spatially arranged in 3-D space. •Crystal structure = lattice (unit cell geometry) + basis (atom, ion, or molecule positions placed on lattice points within the unit cell). •A lattice is used in context when describing crystalline structures, means a 3-D array of points in space. Every lattice point must have identical surroundings. •Unit cell: smallest repetitive volume •Each crystal structure is built by stacking which contains the complete lattice unit cells and placing objects (motifs, pattern of a crystal. A unit cell is chosen basis) on the lattice points: to represent the highest level of geometric symmetry of the crystal structure. It’s the basic structural unit or building block of crystal structure. 7 crystal systems in 3-D 14 crystal lattices in 3-D a, b, and c are the lattice constants 1 a, b, g are the interaxial angles Metallic Crystal Structures (the simplest) •Recall, that a) coulombic attraction between delocalized valence electrons and positively charged cores is isotropic (non-directional), b) typically, only one element is present, so all atomic radii are the same, c) nearest neighbor distances tend to be small, and d) electron cloud shields cores from each other. •For these reasons, metallic bonding leads to close packed, dense crystal structures that maximize space filling and coordination number (number of nearest neighbors). •Most elemental metals crystallize in the FCC (face-centered cubic), BCC (body-centered cubic, or HCP (hexagonal close packed) structures: Room temperature crystal structure Crystal structure just before it melts 2 Recall: Simple Cubic (SC) Structure • Rare due to low packing density (only a-Po has this structure) • Close-packed directions are cube edges. -

Tetrahedral Coordination with Lone Pairs
TETRAHEDRAL COORDINATION WITH LONE PAIRS In the examples we have discussed so far, the shape of the molecule is defined by the coordination geometry; thus the carbon in methane is tetrahedrally coordinated, and there is a hydrogen at each corner of the tetrahedron, so the molecular shape is also tetrahedral. It is common practice to represent bonding patterns by "generic" formulas such as AX4, AX2E2, etc., in which "X" stands for bonding pairs and "E" denotes lone pairs. (This convention is known as the "AXE method") The bonding geometry will not be tetrahedral when the valence shell of the central atom contains nonbonding electrons, however. The reason is that the nonbonding electrons are also in orbitals that occupy space and repel the other orbitals. This means that in figuring the coordination number around the central atom, we must count both the bonded atoms and the nonbonding pairs. The water molecule: AX2E2 In the water molecule, the central atom is O, and the Lewis electron dot formula predicts that there will be two pairs of nonbonding electrons. The oxygen atom will therefore be tetrahedrally coordinated, meaning that it sits at the center of the tetrahedron as shown below. Two of the coordination positions are occupied by the shared electron-pairs that constitute the O–H bonds, and the other two by the non-bonding pairs. Thus although the oxygen atom is tetrahedrally coordinated, the bonding geometry (shape) of the H2O molecule is described as bent. There is an important difference between bonding and non-bonding electron orbitals. Because a nonbonding orbital has no atomic nucleus at its far end to draw the electron cloud toward it, the charge in such an orbital will be concentrated closer to the central atom. -

Oxide Crystal Structures: the Basics
Oxide crystal structures: The basics Ram Seshadri Materials Department and Department of Chemistry & Biochemistry Materials Research Laboratory, University of California, Santa Barbara CA 93106 USA [email protected] Originally created for the: ICMR mini-School at UCSB: Computational tools for functional oxide materials – An introduction for experimentalists This lecture 1. Brief description of oxide crystal structures (simple and complex) a. Ionic radii and Pauling’s rules b. Electrostatic valence c. Bond valence, and bond valence sums Why do certain combinations of atoms take on specific structures? My bookshelf H. D. Megaw O. Muller & R. Roy I. D. Brown B. G. Hyde & S. Andersson Software: ICSD + VESTA K. Momma and F. Izumi, VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data, J. Appl. Cryst. 44 (2011) 1272–1276. [doi:10.1107/S0021889811038970] Crystal structures of simple oxides [containing a single cation site] Crystal structures of simple oxides [containing a single cation site] N.B.: CoO is simple, Co3O4 is not. ZnCo2O4 is certainly not ! Co3O4 and ZnCo2O4 are complex oxides. Graphs of connectivity in crystals: Atoms are nodes and edges (the lines that connect nodes) indicate short (near-neighbor) distances. CO2: The molecular structure is O=C=O. The graph is: Each C connected to 2 O, each O connected to a 1 C OsO4: The structure comprises isolated tetrahedra (molecular). The graph is below: Each Os connected to 4 O and each O to 1 Os Crystal structures of simple oxides of monovalent ions: A2O Cu2O Linear coordination is unusual. Found usually in Cu+ and Ag+. -

Inorganic Chemistry
Inorganic Chemistry Chemistry 120 Fall 2005 Prof. Seth Cohen Some Important Features of Metal Ions Electronic configuration • Oxidation State/Charge. •Size. • Coordination number. • Coordination geometry. • “Soft vs. Hard”. • Lability. • Electrochemistry. • Ligand environment. 1 Oxidation State/Charge • The oxidation state describes the charge on the metal center and number of valence electrons • Group - Oxidation Number = d electron count 3 4 5 6 7 8 9 10 11 12 Size • Size of the metal ions follows periodic trends. • Higher positive charge generally means a smaller ion. • Higher on the periodic table means a smaller ion. • Ions of the same charge decrease in radius going across a row, e.g. Ca2+>Mn2+>Zn2+. • Of course, ion size will effect coordination number. 2 Coordination Number • Coordination Number = the number of donor atoms bound to the metal center. • The coordination number may or may not be equal to the number of ligands bound to the metal. • Different metal ions, depending on oxidation state prefer different coordination numbers. • Ligand size and electronic structure can also effect coordination number. Common Coordination Numbers • Low coordination numbers (n =2,3) are fairly rare, in biological systems a few examples are Cu(I) metallochaperones and Hg(II) metalloregulatory proteins. • Four-coordinate is fairly common in complexes of Cu(I/II), Zn(II), Co(II), as well as in biologically less relevant metal ions such as Pd(II) and Pt(II). • Five-coordinate is also fairly common, particularly for Fe(II). • Six-coordinate is the most common and important coordination number for most transition metal ions. • Higher coordination numbers are found in some 2nd and 3rd row transition metals, larger alkali metals, and lanthanides and actinides. -

Fifty Years of the VSEPR Model R.J
Available online at www.sciencedirect.com Coordination Chemistry Reviews 252 (2008) 1315–1327 Review Fifty years of the VSEPR model R.J. Gillespie Department of Chemistry, McMaster University, Hamilton, Ont. L8S 4M1, Canada Received 26 April 2007; accepted 21 July 2007 Available online 27 July 2007 Contents 1. Introduction ........................................................................................................... 1315 2. The VSEPR model ..................................................................................................... 1316 3. Force constants and the VSEPR model ................................................................................... 1318 4. Linnett’s double quartet model .......................................................................................... 1318 5. Exceptions to the VSEPR model, ligand–ligand repulsion and the ligand close packing (LCP) model ........................... 1318 6. Analysis of the electron density.......................................................................................... 1321 7. Molecules of the transition metals ....................................................................................... 1325 8. Teaching the VSEPR model ............................................................................................. 1326 9. Summary and conclusions .............................................................................................. 1326 Acknowledgements ................................................................................................... -

Searching Coordination Compounds
CAS ONLINEB Available on STN Internationalm The Scientific & Technical Information Network SEARCHING COORDINATION COMPOUNDS December 1986 Chemical Abstracts Service A Division of the American Chemical Society 2540 Olentangy River Road P.O. Box 3012 Columbus, OH 43210 Copyright O 1986 American Chemical Society Quoting or copying of material from this publication for educational purposes is encouraged. providing acknowledgment is made of the source of such material. SEARCHING COORDINATION COMPOUNDS prepared by Adrienne W. Kozlowski Professor of Chemistry Central Connecticut State University while on sabbatical leave as a Visiting Educator, Chemical Abstracts Service Table of Contents Topic PKEFACE ............................s.~........................ 1 CHAPTER 1: INTRODUCTION TO SEARCHING IN CAS ONLINE ............... 1 What is Substructure Searching? ............................... 1 The Basic Commands .............................................. 2 CHAPTEK 2: INTKOOUCTION TO COORDINATION COPPOUNDS ................ 5 Definitions and Terminology ..................................... 5 Ligand Characteristics.......................................... 6 Metal Characteristics .................................... ... 8 CHAPTEK 3: STKUCTUKING AND REGISTKATION POLICIES FOR COORDINATION COMPOUNDS .............................................11 Policies for Structuring Coordination Compounds ................. Ligands .................................................... Ligand Structures........................................... Metal-Ligand -

Coordination Number 2
Coordination Numbers and Geometry Page 1 of 11 Coordination Numbers and Geometry Lecture 2. CHEM1902 (C 10K) Coordination Chemistry The total number of points of attachment to the central element is termed the coordination number and this can vary from 2 to as many as 16, but is usually 6. In simple terms, the coordination number of a complex is influenced by the relative sizes of the metal ion and the ligands and by electronic factors, such as charge which is dependent on the electronic configuration of the metal ion. These competing effects are described by the term ionic potential which is defined as the charge to radius ratio. Based on this, it can be seen that the bigger the charge on the central ion, the more attraction there will be for negatively charged ligands, however at the same time, the bigger the charge the smaller the ion becomes which then limits the number of groups able to coordinate. Coordination Number 2 + The linear [Ag(NH 3)2] ion This arrangement is not very common for first row transition metal ion complexes and some of the best known examples are for Silver(I). In this case we have a low charge and an ion at the right hand side of the d- block indicating smaller size For instance, a method often employed for the detection of chloride ions involves the formation of the linear diamminesilver(I) complex. The first step is: Ag+ + Cl- → AgCl (white ppt) and to ensure that the precipitate is really the chloride salt, two further Although [Ag(en)]ClO 4 involves a tests must be done: normally bidentate ligand, in this case the structure is polymeric and AgCl + 2 NH [Ag(NH ) ]+ 3 → 3 2 the silver ion still retains a CN=2. -

Lecture 2--2016
Chem 462 Lecture 2--2016 z [MLnXm] Classification of Ligands Metal Oxidation States Electron Counting Overview of Transition Metal Complexes 1.The coordinate covalent or dative bond applies in L:M 2.Lewis bases are called LIGANDS—all serve as σ-donors some are π-donors as well, and some are π-acceptors 3. Specific coordination number and geometries depend on metal and number of d-electrons 4. HSAB theory useful z [MLnXm] a) Hard bases stabilize high oxidation states b) Soft bases stabilize low oxidation states Classification of Ligands: The L, X, Z Approach Malcolm Green : The CBC Method (or Covalent Bond Classification) used extensively in organometallic chemistry. L ligands are derived from charge-neutral precursors: NH3, amines, N-heterocycles such as pyridine, PR3, CO, alkenes etc. X ligands are derived from anionic precursors: halides, hydroxide, alkoxide alkyls—species that are one-electron neutral ligands, but two electron 4- donors as anionic ligands. EDTA is classified as an L2X4 ligand, features four anions and two neutral donor sites. C5H5 is classified an L2X ligand. Z ligands are RARE. They accept two electrons from the metal center. They donate none. The “ligand” is a Lewis Acid that accepts electrons rather than the Lewis Bases of the X and L ligands that donate electrons. Here, z = charge on the complex unit. z [MLnXm] octahedral Square pyramidal Trigonal bi- pyramidal Tetrahedral Range of Oxidation States in 3d Transition Metals Summary: Classification of Ligands, II: type of donor orbitals involved: σ; σ + π; σ + π*; π+ π* Ligands, Classification I, continued Electron Counting and the famous Sidgewick epiphany Two methods: 1) Neutral ligand (no ligand carries a charge—therefore X ligands are one electron donors, L ligands are 2 electron donors.) 2) Both L and X are 2-electron donor ligand (ionic method) Applying the Ionic or 2-electron donor method: Oxidative addition of H2 to chloro carbonyl bis triphenylphosphine Iridium(I) yields Chloro-dihydrido-carbonyl bis-triphenylphosphine Iridium(III). -
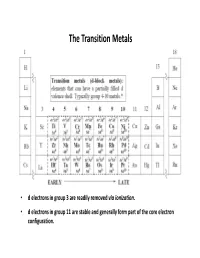
The Transition Metals
The Transition Metals • d electrons in group 3 are readily removed via ionization . • d electrons in group 11 are stable and generally form part of the core electron configuration. Transition Metal Valence Orbitals • nd orbitals • (n + 1)s and (n + 1)p orbitals • 2 2 2 dx -dy and dz (e g) lobes located on the axes • dxy, dxz, dyz lobes (t 2g) located between axes • orbitals oriented orthogonal wrt each other creating unique possibilities for ligand overlap. • Total of 9 valence orbitals available for bonding (2 x 9 = 18 valence electrons!) • For an σ bonding only Oh complex, 6 σ bonds are formed and the remaining d orbitals are non-bonding . • It's these non-bonding d orbitals that give TM complexes many of their unique properties • for free (gas phase) transition metals: (n+1)s is below (n)d in energy (recall: n = principal quantum #). • for complexed transition metals: the (n)d levels are below the (n+1)s and thus get filled first. (note that group # = d electron count) • for oxidized metals, subtract the oxidation state from the group #. Geometry of Transition Metals Coordination Geometry – arrangement of ligands around metal centre Valence Shell Electron Pair Repulsion (VSEPR) theory is generally not applicable to transition metals complexes (ligands still repel each other as in VSEPR theory) For example, a different geometry would be expected for metals of different d electron count 3+ 2 [V(OH 2)6] d 3+ 4 [Mn(OH 2)6] d all octahedral geometry ! 3+ 6 [Co(OH 2)6] d Coordination geometry is , in most cases , independent of ground state electronic configuration Steric: M-L bonds are arranged to have the maximum possible separation around the M. -

Isomers and Coordination Geometries Chapter 9
Coordination Chemistry II: Isomers and Coordination Geometries Chapter 9 Monday, November 16, 2015 A Real World Example of Stuff from Class! Isomerism Coordination complexes often have a variety of isomeric forms Structural Isomers Molecules with the same numbers of the same atoms, but in different arrangements. Isomers generally have distinct physical and chemical properties. One isomer may be a medicine while another is a poison. Type 1: Structural isomers differ in how the atoms are connected. As a result, they have different chemical formulas. e.g., C3H8O 1-propanol 2-propanol methoxyethane m.p. -127°C m.p. -89°C m.p. -139°C b.p. 97°C b.p. 83°C b.p. 8°C Structural Isomers Structural (or constitutional) isomers are molecules with the same kind and number of atoms but with different bond arrangements In coordination complexes there are four types of structural isomers: • hydrate (solvent) isomerism occurs when water (or another solvent) can appear within the primary or secondary coordination sphere of a metal ion Cr H O Cl CrCl H O Cl H O 2 6 3 2 5 2 2 violet crystals blue-green crystals CrCl H O Cl 2H O CrCl H O 3H O 2 2 4 2 3 2 3 2 dark green crystals yellow-green crystals • ionization isomers afford different anions and cations in solution Co NH SO NO Co NH NO SO 3 5 4 3 3 5 3 4 Structural Isomers In coordination complexes there are four types of structural isomers • coordination isomerism occurs when ligands can be distributed differently between two or more metals • linkage isomerism occurs when a ligand can bind in different ways to a metal Stereoisomers The second major class of isomer. -

Body Centered Cubic Structure
CHAPTER 3: CRYSTAL STRUCTURES & PROPERTIES Metallic Crystals • tend to be densely packed. • have several reasons for dense packing: -Typically, only one element is present, so all atomic radii are the same. -Metallic bonding is not directional. -Nearest neighbor distances tend to be small in order to lower bond energy. • have the simplest crystal structures. We will look at three such structures... Cubic Unit Cells a 2R SIMPLE CUBIC BODY CENTERED FACE CENTERED STRUCTURE (SC) CUBIC STRUCTURE (BCC) CUBIC STRUCTURE (FCC) Simple Cubic (SC) Structure • Coordination number is the number of nearest neighbors • Linear density (LD) is the number of atoms per unit length along a specific crystallographic direction . a3 . a2 a1 . Coordination number = 6 LD110 = 1 atoms/2√2 R LD100= 1 atoms/2R • Rare due to poor packing (only Po [84] has this structure) • Close-packed directions are cube edges. Atomic Packing Factor (APF) • APF for a simple cubic structure = 0.52 Body Centered Cubic (BCC) Structure • Coordination number = 8 • Close packed directions are cube diagonals: LD110= 1 atom/(4R√(2/3))=1/(2R√8/3) LD001 = 1 atom/(4R/√3))= 1/(2R √4/3) LD111 = 2 atoms/4R = 1/(2R) • Unit cell contains: R 1 + 8 x 1/8 = 2 atoms/unit cell a • APF = 0.68: 8 Face-Centered Cubic (FCC) Structure • Coordination number = 12 • Close packed directions are face diagonals: LD110= 2 atom/(4R)= 1/2R LD001 = 1 atom/(2R√2))= 1/(2R √2) LD111 = 1 atoms/4R = 1/(2R √6 ) • Unit cell contains : a 6 x 1/2 + 8 x 1/8 = 4 atoms/unit cell • APF = 0.74 Hexagonal Close-Packed (HCP) Structure • ABAB..