Dáil Éireann
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
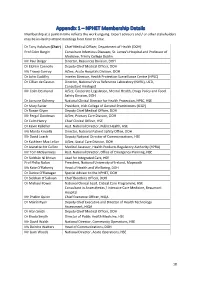
Appendix 1 – NPHET Membership Details Membership at a Point in Time Reflects the Work Ongoing
Appendix 1 – NPHET Membership Details Membership at a point in time reflects the work ongoing. Expert advisors and / or other stakeholders may be invited to attend meetings from time to time. Dr Tony Holohan (Chair) Chief Medical Officer, Department of Health (DOH) Prof Colm Bergin Consultant Infectious Diseases, St. James’s Hospital and Professor of Medicine, Trinity College Dublin Mr Paul Bolger Director, Resources Division, DOH Dr Eibhlin Connolly Deputy Chief Medical Officer, DOH Ms Tracey Conroy A/Sec, Acute Hospitals Division, DOH Dr John Cuddihy Interim Director, Health Protection Surveillance Centre (HPSC) Dr Cillian de Gascun Director, National Virus Reference Laboratory (NVRL), UCD, Consultant Virologist Mr Colm Desmond A/Sec, Corporate Legislation, Mental Health, Drugs Policy and Food Safety Division, DOH Dr Lorraine Doherty National Clinical Director for Health Protection, HPSC, HSE Dr Mary Favier President, Irish College of General Practitioners (ICGP) Dr Ronan Glynn Deputy Chief Medical Officer, DOH Mr Fergal Goodman A/Sec, Primary Care Division, DOH Dr Colm Henry Chief Clinical Officer, HSE Dr Kevin Kelleher Asst. National Director, Public Health, HSE Ms Marita Kinsella Director, National Patient Safety Office, DOH Mr David Leach Deputy National Director of Communications, HSE Dr Kathleen Mac Lellan A/Sec, Social Care Division, DOH Dr Jeanette Mc Callion Medical Assessor, Health Products Regulatory Authority (HPRA) Mr Tom McGuinness Asst. National Director, Office of Emergency Planning, HSE Dr Siobhán Ní Bhrian Lead for -

1 National Public Health Emergency Team – COVID-19 Meeting Note
National Public Health Emergency Team – COVID-19 Meeting Note – Standing Meeting Date and Time Thursday 28th May 2020, (Meeting 33) at 10:00am Location Department of Health, Miesian Plaza, Dublin 2 Chair Dr Tony Holohan, Chief Medical Officer, DOH Dr Kevin Kelleher, Assistant National Director, Public Health, HSE Mr Liam Woods, National Director, Acute Operations, HSE Dr Darina O’Flanagan, Special Advisor to the NPHET Prof Philip Nolan, President, National University of Ireland, Maynooth and Chair of the Irish Epidemiological Modelling Advisory Group (IEMAG) Dr Lorraine Doherty, National Clinical Director Health Protection, HSE Dr Cillian de Gascun, Laboratory Director, NVRL and Expert Advisory Group (EAG) Chair Mr David Leach, Communications, HSE Dr Mary Favier, President, Irish College of General Practitioners (ICGP) Mr Phelim Quinn, Chief Executive Officer, HIQA Dr Michael Power, Consultant in Anaesthetics / Intensive Care Medicine, Beaumont Hospital Dr Máirín Ryan, Deputy Chief Executive and Director of Health Technology Assessment, HIQA Dr Ronan Glynn, Deputy Chief Medical Officer, DOH Members via Dr Alan Smith, Deputy Chief Medical Officer, DOH videoconference Dr Eibhlin Connolly, Deputy Chief Medical Officer, DOH Dr Siobhan O’Sullivan, Chief Bioethics Officer, DOH Ms Tracey Conroy, Assistant Secretary, Acute Hospitals Policy Division, DOH Mr Fergal Goodman, Assistant Secretary, Primary Care Division, DOH Mr Colm Desmond, Assistant Secretary, Corporate Legislation, Mental Health, Drugs Policy and Food Safety Division, DOH Mr Paul -
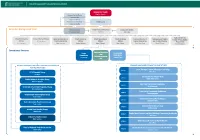
Executive Management Team Operational Services
Executive Management Team and Operational Services Minister for Health Stephen Donnelly Board Committees Audit and Risk People and Culture Performance and Delivery HSE Board Safety and Quality Executive Management Team Internal Audit Chief Executive Officer Corporate Affairs Dr Geraldine Smith Paul Reid John Kelly National Director Chief Information Chief Clinical Officer National Director of Chief Financial Chief Operations Chief Strategy National Director of National Lead Testing COVID-19 Vaccination Officer Dr Colm Henry Communications Officer Officer Officer Human Resources and Contact Tracing Programme Fran Thompson Mark Brennock Stephen Mulvany Anne O’Connor Dean Sullivan Anne Marie Hoey Niamh O’Beirne Damien McCallion Operational Services National Schemes Acute Community and Operations Operations Reimbursements Liam Woods Yvonne O’Neill TBC community healthcare organisations chief officers hospital groups ceos Cavan, Donegal, Leitrim, Monaghan and Sligo area 1 RCSI Hospital Group John Hayes Ian Carter Community Healthcare West area 2 Dublin Midlands Hospital Group Breda Crehan Roche Trevor O’Callaghan Mid West Community Healthcare area 3 University of Limerick Hospitals Group Maria Bridgeman Prof Colette Cowan Cork, Kerry, Community Healthcare area 4 Michael Fitzgerald South/South West Hospital Group Gerry O’Dwyer South East Community Healthcare area 5 Kate Killeen White Saolta University Health Care Group Tony Canavan area 6 Community Healthcare East Martina Queally Ireland East Hospital Group Declan Lyons area 7 Dublin South, Kildare and West Wicklow Community Healthcare Ann O’Shea Children’s Health Ireland Eilish Hardiman area 8 Midlands Louth Community Healthcare Des O’Flynn Head of National Ambulance Service Dublin North City and County Community Healthcare area 9 Robert Morton Mellany McLoone. -

High Level Task Force on COVID-19 Vaccination
High Level Task Force on COVID-19 Vaccination 8 February 2021 Meeting Updates, decisions and actions from meeting High Level Taskforce on COVID-19 Vaccination | 8 February 2021 Meeting High Level Task Force on COVID-19 Vaccination Monday 8 February 2021 14:00 Updates, decisions and actions arising from meeting 1. Attendees A. Members in attendance B. Additional attendees in support Prof Brian MacCraith, Task Force Chair ii. In Attendance Liz Canavan, Chair, SOG on COVID-19 Sean Bresnan, National Director of Procurement, HSE Fergal Goodman, Assistant Secretary, Health Dr Ronan Glynn, Deputy CMO, DOH Protection Division, DOH Dr Colm Henry, Chief Clinical Officer, HSE Deirdre Watters, Head of Communications, DOH Rachel Kenna, Chief Nursing Officer, DOH Elizabeth Headon, Programme Communications Barry Lowry, Chief Information Officer, OGCIO Dr Lucy Jessop, SRO WS2, Director, NIO, HSE Derek McCormack, Expert on Cold Chain Logistics Paul Flanagan, SRO WS3 Dermot Mulligan, Assistant Secretary, Innovation David Walsh, SRO WS4 and Investment Division, DETE Lorraine Nolan, Chief Executive, HPRA Dr John Cuddihy, SRO WS5 Dr Nuala O'Connor, ICGP Fran Thompson, SRO WS6 Dalton Philips, Chief Executive Officer, DAA David Leach, SRO WS7 Paul Quinn, Government CPO and CEO, OGP Andrew Byrne, Immunisation and Infectious Diseases Policy, DOH Paul Reid, Chief Executive Officer, HSE Deirdre McNamara, General Manager, Quality & Patient Safety, Acute Hospitals Division, HSE Martin Shanahan, Chief Executive Officer, IDA Minister Stephen Donnelly, Minister for Health Derek Tierney, Programme Director iii. In partial attendance Additional attendees in support Frances McNamara, Senior Operations and Corporate, HSE i. Task Force Secretariat iv. Programme support Kate Waterhouse, Task Force Secretariat Yvonne Mowlds (PWC), Programme Office Apologies: Prof Karina Butler, Chair, NIAC 2 High Level Taskforce on COVID-19 Vaccination | 8 February 2021 Meeting 2. -

Link to CAO Annual Report 2020
CHIEF ACADEMIC OFFICERS IRELAND ANNUAL REPORT 2020 C A O G R O U P | A N N U A L R E P O R T 0 1 CHIEF ACADEMIC OFFICERS Professor Paul Burke, MD FRCSI, Associate Professor, Vice Dean (Health Sciences) - University of Limerick, Adjunct Professor of Surgery, UL GEMS, Chief Academic Officer - UL Hospital Group Professor Arnie Hill, Chair of Surgery - RCSI. Chief Academic Officer, RCSI Hospitals Professor Joseph Keane, Consultant Respiratory Physician, Head of Clinical Medicine – St James’s Hospital, Dublin Midlands Hospital Group HSE Professor Timothy Lynch, Consultant Neurologist - Mater Misericordiae Hospital, VP Health Affairs - UCD, Chief Academic Officer –Ireland East Hospital Group Professor Anthony O'Regan, Consultant Physician - Galway University Hospital, Chief Academic Officer –Saolta University Health Care Group Professor Owen Smith, UCD Professor of Paediatric and Adolescent Medicine, Consultant Paediatric Haematologist at Children’s Health Ireland at Crumlin, Chief Academic Lead to the Children's Hospital Group Professor Helen Whelton, Head of College of Medicine and Health - University College Cork, Chief Academic Officer –HSE South, South West Hospital Group C A O G R O U P | A N N U A L R E P O R T 0 2 A LETTER FROM THE CAO GROUP As Chief Academic Officers (CAOs) we act as a link between our hospital groups and universities – ensuring innovation, research and teaching remain at the forefront of Irish healthcare. As COVID-19 made itself known throughout Ireland in 2020 we begun to harness our resources to focus on areas that needed urgent attention. In the initial stages of the COVID-19 pandemic the CAO’s worked with their partner universities, hospital groups and industry to assist with laboratory diagnostics. -

August 2020 College of Medicine and Health, UCC
CoMH eNEWS Issue 37 | August 2020 College of Medicine and Health, UCC Navigate stories Welcome to our latest CoMH online newsletter New microbiome research of practical value to patients Congratulations to Virtual Conferrings » Graduates of our Medical and Summer Conferrings Infant & Dentistry Researchers awarded ¤500,000 in HRB funding Two awards for ‘Outstanding Junior Scientist’ Dr Erin Harris MSc in Human Anatomy open on PAC Research » HRB Funding » CRF-C to play lead role in WHO COVID-19 Solidarity Trial Other news CoMH eNews is intended for circulation among staff and students of the College of Medicine and Health, UCC. Extracts from CoMH eNews should not be published without the permission of the editor. Awards » Courses » Coronavirus Response » For info and submissions email: [email protected] Start reading CoMH eNews CoMH eNEWS Message from Head of College Issue 37 | August 2020 Our researchers continue to be extremely partner of APC, discovers, develops and ¤16m study ‘SOPHIA’ to better understand active in the race to understand and markets probiotics (live bacteria), which can obesity and optimise future treatments. mitigate COVID-19. The director of CRF-C, improve gut health in animals and humans. professor Joe Eustace was nominated by the CoMH researchers were successful in 3 Minister of Health to be the National Chief I’d like to congratulate Professor Chris Lynch, separate H2020 funding initiatives. APC investigator for the WHO Solidarity Clinical professor and consultant in Restorative Microbiome Ireland researchers Dr Gerard Trial in Ireland, with the Department of Health Dentistry who is a recipient of Ireland’s Clarke and Professor John F. -

Statement from the National Public Health Emergency Team - Wednesday 30 September
Statement from the National Public Health Emergency Team - Wednesday 30 September From Department of Health Published at 30 September 2020 Last updated 1 October 2020 • 1. Hospital statistics • 2. Gender of patients • 3. Age range affected • 4. How COVID-19 is spreading • 5. Hospitalised cases by age group • 6. Cases by county The Health Protection Surveillance Centre has today been informed that 1 person with COVID-19 has died. There has now been a total of 1,804 COVID-19 related deaths in Ireland. As of midnight Tuesday 29 September, the HPSC has been notified of 429 confirmed cases of COVID-19. There has now been a total of 36,155* confirmed cases of COVID-19 in Ireland. (*Validation of data at the HPSC has resulted in the denotification of 14 confirmed cases. The figure of 36,155 confirmed cases reflects this.) Of the cases notified today: • 203 are men and 226 are women • 65% are under 45 years of age • 45% are confirmed to be associated with outbreaks or are close contacts of a confirmed case • 77 cases have been identified as community transmission • 189 cases are in Dublin, 60 in Cork, 31 in Donegal, 28 in Galway, 18 in Kildare, 15 in Wicklow, 15 in Clare, 12 in Limerick, 9 in Meath, 8 in Louth, 7 in Cavan, 7 in Longford, 6 in Laois, 5 in Offaly, 5 in Westmeath, with the remaining 14 cases in 8 counties The HSE is working to identify any contacts the patients may have had to provide them with information and advice to prevent further spread. -

5Th NATIONAL
5th NATIONAL PATIENT SAFETY CONFERENCE Aviva Stadium, Dublin 4 Thursday, 12th November 2015 PROGRA MME 8.30 – 9.15 S E S S I O N 2 REGISTRATION (tea/coffee) 11.20 – 12.30 Good practice presentations 9.15 – 9.35 Opening address BREAK-OUT SESSION A (Main Auditorium – Level 2) Mr Leo Varadkar, TD, Minister for Health Chair: Ms Triona Fortune, Deputy CEO, International Society for Quality in Health Care S E S S I O N 1 Plenary Chair: Presentation 1 Dr Tony Holohan, Chief Medical Officer Benzodiazepines and hypnotics use in an acute mental health service Presenter: Ms Ailish Young, Pharmacist, St Patrick’s Mental Health Services, and 9.35 – 10.05 Dr Adam Kavanagh, Nurse Practice Development Coordinator, St Patrick’s Mental The future of patient safety and how to Health Services broaden from the current emphasis on the Presentation 2 study of past harms to reliability and NOCA National ICU Audit first steps – The Mater journey preparedness Presenters: Ms Mary Baggot, National Office of Clinical Audit and Ms Elaine Ms Helen Crisp, Assistant Director of Langan, Critical Care Audit Nurse, Mater Misericordiae University Hospital Research, The Health Foundation, London Presentation 3 Improving patient safety by early recognition and intervention for urinary 10.05 – 10.35 retention in the Rotunda Hospital re-audit from January 2014 to July 2014 Learning from the evidence about what Presenters: Ms Cinny Cusack, Physiotherapy Manager and Ms Mary O’Reilly, works in improving quality and safety in Practice Development Coordinator, Rotunda Hospital, Dublin 1 healthcare Presentation 4 Professor Mary Dixon-Woods, Professor of The Shocking Stocking Audit…. -

Transforming Healthcare Education
Annual Report 16/17 Transforming Healthcare Education RCSI DEVELOPING HEALTHCARE LEADERS WHO MAKE A DIFFERENCE WORLDWIDE 2 1 CONTENTS Governance 06 President’s Review 08 Strategic Plan Accomplishments 13 Council Members 14 Chief Executive’s Review 16 Senior Management Team 21 Milestones and Achievements 22 Surgical Affairs 24 Research and Innovation 32 Healthcare Management 38 Healthcare Management Overview 40 Institute of Leadership 42 Education, Teaching and Learning 44 Faculty of Medicine and Health Sciences 46 School of Medicine 50 School of Pharmacy 54 School of Physiotherapy 56 School of Nursing and Midwifery 58 School of Postgraduate Studies 60 Faculty of Dentistry 62 Faculty of Radiologists 64 Faculty of Nursing and Midwifery 66 Faculty of Sports & Exercise Medicine RCPI and RCSI 68 Irish Institute of Pharmacy 70 International Campuses 72 RCSI Development - Alumni Relations, Fellows and Members 78 RCSI People 82 Human Resources at RCSI 84 Student Experience 86 RCSI in the Community 88 Initiatives in Ireland 90 Global initiatives 93 Finance 96 Appendices 102 The RCSI Annual Report 2016/2017 covers the College’s operations in Ireland during the period 1st July 2016 – 30th June 2017. 2 RCSI ANNUAL REPORT 2016-2017 MILESTONE ANNIVERSARIES 10year 10year 10year 20year Anniversary of Anniversary Anniversary of School Anniversary of Graduate Entry of RCSI Dubai of Postgraduate Penang Medical Medicine in Ireland Studies College Malaysia WORLD RANKINGS RCSI IN NUMBERS INVESTMENT Top Times2% Higher Education World University Rankings 80minvested -

1 National Public Health Emergency Team – COVID-19 Meeting Note
National Public Health Emergency Team – COVID-19 Meeting Note – Standing meeting Date and Time Wednesday 28th April 2021, (Meeting 86) at 10:00am Location Department of Health, Miesian Plaza, Dublin 2 Chair Dr Tony Holohan, Chief Medical Officer, DOH Dr Ronan Glynn, Deputy Chief Medical Officer, DOH Dr Kevin Kelleher, Assistant National Director, Public Health, HSE Prof Philip Nolan, President, National University of Ireland, Maynooth and Chair of the Irish Epidemiological Modelling Advisory Group (IEMAG) Dr Cillian de Gascun, Laboratory Director, NVRL Dr Máirín Ryan, Deputy Chief Executive and Director of HTA, HIQA Prof Colm Bergin, Consultant in Infectious Diseases, St James’s Hospital Dr Siobhán Ní Bhriain, Lead for Integrated Care, HSE Dr Eibhlín Connolly, Deputy Chief Medical Officer, DOH Dr Mary Favier, Immediate past president of the ICGP, Covid-19 advisor Dr Michael Power, Consultant in Anaesthetics / Intensive Care Medicine, Beaumont Hospital Ms Rachel Kenna, Chief Nursing Officer, DOH Ms Tracey Conroy, Assistant Secretary, Acute Hospitals Policy Division, DOH Dr Lorraine Doherty, National Clinical Director Health Protection, HSE Dr Colette Bonner, Deputy Chief Medical Officer, DOH Ms Yvonne O’Neill, National Director, Community Operations, HSE Mr Phelim Quinn, Chief Executive Officer, HIQA Members via Prof Mark Ferguson, Director General, Science Foundation Ireland, and Chief Scientific Adviser to the Government videoconference1 of Ireland, SFI Mr Greg Dempsey, Deputy Secretary, Governance and Performance Division, DOH Mr Fergal -

1 National Public Health Emergency Team – COVID-19 Meeting Note
National Public Health Emergency Team – COVID-19 Meeting Note – Standing meeting Date and Time Thursday 8th October 2020, (Meeting 58) at 10:00am Location Department of Health, Miesian Plaza, Dublin 2 Chair Dr Tony Holohan, Chief Medical Officer, DOH Dr Ronan Glynn, Deputy Chief Medical Officer, DOH Dr Kevin Kelleher, Assistant National Director, Public Health, HSE Prof Philip Nolan, President, National University of Ireland, Maynooth and Chair of the Irish Epidemiological Modelling Advisory Group (IEMAG) Dr Cillian de Gascun, Laboratory Director, NVRL and Expert Advisory Group (EAG) Chair Dr Máirín Ryan, Deputy Chief Executive and Director of HTA, HIQA Dr John Cuddihy, Interim Director, HSE HPSC Dr Michael Power, Consultant in Anaesthetics / Intensive Care Medicine, Beaumont Hospital Dr Siobhán Ní Bhriain, Lead for Integrated Care, HSE Dr Eibhlín Connolly, Deputy Chief Medical Officer, DOH Dr Mary Favier, Immediate past president of the ICGP, Covid-19 advisor Ms Tracey Conroy, Assistant Secretary, Acute Hospitals Policy Division, DOH Dr Siobhán O’Sullivan, Chief Bioethics Officer, DOH Members via Dr Colette Bonner, Deputy Chief Medical Officer, DOH videoconference Mr Colm Desmond, Assistant Secretary, Corporate Legislation, Mental Health, Drugs Policy and Food Safety Division, DOH Mr Phelim Quinn, Chief Executive Officer, HIQA Dr Darina O’Flanagan, Special Advisor to the NPHET Mr Fergal Goodman, Assistant Secretary, Primary Care Division, DOH Dr Breda Smyth, Public Health Specialist, HSE Ms Yvonne O’Neill, National Director, Community -

1 National Public Health Emergency Team – COVID-19 Meeting Note
National Public Health Emergency Team – COVID-19 Meeting Note – Standing Meeting Date and Time Tuesday, 12th May 2020 (Meeting 30) at 10am Location Department of Health, Miesian Plaza, Dublin 2 Chair Dr Tony Holohan, Chief Medical Officer, DOH Dr Kevin Kelleher, Assistant National Director, Public Health, HSE Mr Liam Woods, National Director, Acute Operations, HSE Mr David Walsh, National Director, Community Operations, HSE Dr Darina O’Flanagan, Special Advisor to the NPHET Prof Philip Nolan, President, National University of Ireland, Maynooth and Chair of the Irish Epidemiological Modelling Advisory Group (IEMAG) Dr Lorraine Doherty, National Clinical Director Health Protection, HSE Dr Cillian de Gascun, Laboratory Director, NVRL and Expert Advisory Group (EAG) Chair Dr Darina O’Flanagan, Special Advisor to the NPHET Mr David Leach, Communications, HSE Dr Mary Favier, President, Irish College of General Practitioners (ICGP) Mr Phelim Quinn, Chief Executive Officer, HIQA Dr Michael Power, Consultant in Anaesthetics / Intensive Care Medicine, Beaumont Hospital Dr Máirín Ryan, Deputy Chief Executive and Director of Health Technology Assessment, Members via HIQA videoconference Prof Colm Bergin, Consultant in Infectious Diseases, St James’s Hospital Dr Ronan Glynn, Deputy Chief Medical Officer, DOH Dr Alan Smith, Deputy Chief Medical Officer, DOH Dr Eibhlin Connolly, Deputy Chief Medical Officer, DOH Dr Siobhan O’Sullivan, Chief Bioethics Officer, DOH Ms Tracey Conroy, Assistant Secretary, Acute Hospitals Division, DOH Mr Fergal Goodman,