High Level Task Force on COVID-19 Vaccination
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
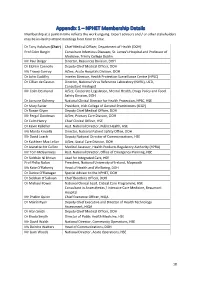
Appendix 1 – NPHET Membership Details Membership at a Point in Time Reflects the Work Ongoing
Appendix 1 – NPHET Membership Details Membership at a point in time reflects the work ongoing. Expert advisors and / or other stakeholders may be invited to attend meetings from time to time. Dr Tony Holohan (Chair) Chief Medical Officer, Department of Health (DOH) Prof Colm Bergin Consultant Infectious Diseases, St. James’s Hospital and Professor of Medicine, Trinity College Dublin Mr Paul Bolger Director, Resources Division, DOH Dr Eibhlin Connolly Deputy Chief Medical Officer, DOH Ms Tracey Conroy A/Sec, Acute Hospitals Division, DOH Dr John Cuddihy Interim Director, Health Protection Surveillance Centre (HPSC) Dr Cillian de Gascun Director, National Virus Reference Laboratory (NVRL), UCD, Consultant Virologist Mr Colm Desmond A/Sec, Corporate Legislation, Mental Health, Drugs Policy and Food Safety Division, DOH Dr Lorraine Doherty National Clinical Director for Health Protection, HPSC, HSE Dr Mary Favier President, Irish College of General Practitioners (ICGP) Dr Ronan Glynn Deputy Chief Medical Officer, DOH Mr Fergal Goodman A/Sec, Primary Care Division, DOH Dr Colm Henry Chief Clinical Officer, HSE Dr Kevin Kelleher Asst. National Director, Public Health, HSE Ms Marita Kinsella Director, National Patient Safety Office, DOH Mr David Leach Deputy National Director of Communications, HSE Dr Kathleen Mac Lellan A/Sec, Social Care Division, DOH Dr Jeanette Mc Callion Medical Assessor, Health Products Regulatory Authority (HPRA) Mr Tom McGuinness Asst. National Director, Office of Emergency Planning, HSE Dr Siobhán Ní Bhrian Lead for -

Dáil Éireann
Vol. 1006 Wednesday, No. 7 12 May 2021 DÍOSPÓIREACHTAÍ PARLAIMINTE PARLIAMENTARY DEBATES DÁIL ÉIREANN TUAIRISC OIFIGIÚIL—Neamhcheartaithe (OFFICIAL REPORT—Unrevised) Insert Date Here 12/05/2021A00100Ábhair Shaincheisteanna Tráthúla - Topical Issue Matters 884 12/05/2021A00175Saincheisteanna Tráthúla - Topical Issue Debate 885 12/05/2021A00200Digital Hubs ����������������������������������������������������������������������������������������������������������������������������������������������������������885 12/05/2021B00350Hospital Waiting Lists 887 12/05/2021C00400Special Educational Needs 891 12/05/2021E00300Harbours and Piers 894 12/05/2021F00600Companies (Protection of Employees’ Rights in Liquidations) Bill 2021: Second Stage [Private Members] 897 12/05/2021S00500Ceisteanna ó Cheannairí - Leaders’ Questions 925 12/05/2021W00500Ceisteanna ar Reachtaíocht a Gealladh - Questions on Promised Legislation 935 12/05/2021AA00800Pensions (Amendment) (Transparency in Charges) Bill 2021: First Stage 945 12/05/2021AA01700Health (Regulation of Termination of Pregnancy) (Foetal Pain Relief) Bill 2021: First Stage 946 12/05/2021BB00900Ministerial Rota for Parliamentary Questions: Motion -

Dáil Éireann
DÁIL ÉIREANN AN COMHCHOISTE UM SHLÁINTE JOINT COMMITTEE ON HEALTH Dé Céadaoin, 18 Nollaig 2019 Wednesday, 18 December 2019 Tháinig an Comhchoiste le chéile ag 10 a.m. The Joint Committee met at 10 a.m. Comhaltaí a bhí i láthair/Members present: Teachtaí Dála/Deputies Seanadóirí/Senators Bernard J. Durkan, Colm Burke. Alan Kelly, Margaret Murphy O’Mahony, Kate O’Connell, Louise O’Reilly. I láthair/In attendance: Deputy Bríd Smith. Teachta/Deputy Michael Harty sa Chathaoir/in the Chair. 1 JH The joint committee met in private session until 10.13 a.m. Royal College of Obstetricians and Gynaecologists Independent Expert Panel Review into Cervical Screening: Discussion Chairman: The purpose of the meeting is to examine the findings and recommendations of the Royal College of Obstetricians and Gynaecologists, RCOG, independent expert panel re- view into cervical screening in cases of cervical cancer in Ireland between 2008 and 2018. On behalf of the committee, I welcome the expert panel lead assessors: Professor Henry Kitchener, lead assessor, and Dr. Patrick Walker, deputy lead assessor, ROCG. Attending on behalf of the Department of Health are Dr. Tony Holohan, chief medical of- ficer; Ms Tracey Conroy, assistant secretary with responsibility for acute hospitals; Dr. Ronan Glynn, deputy chief medical officer; and Ms Celeste O’Callaghan, principal officer on the- Cer vicalCheck project team. I welcome from the HSE Mr. Damien McCallion, interim national director of the national screening service, Dr. Colm Henry, chief clinical officer, Ms Celine Fitzgerald, interim CEO of the national screening service, Dr. Lorraine Doherty, clinical di- rector of CervicalCheck, and Dr. -

What Are the Alternatives to Our Broken Direct Provision System?
Position Paper What are the alternatives to our broken Direct Provision system? February 2019 Direct provision is already a chapter in Ireland’s dark history of institutional living. More of the same fails us all. Direct provision is broken, condemned by politicians across the political spectrum, international organisations and, most importantly, by residents themselves. Geoffrey Shannon, the special rapporteur on child protection, called for it to be abolished in his recent annual report. Its failures have been vividly demonstrated through Melatu Uche Okorie’s book Hostel People and the TV show Taken Down. So how do we end direct provision, what is the alternative and how do we get there? Minister of State for Justice David Stanton repeatedly says he has not heard of a better system. These are some ideas. In short, we need to shift to long-term, strategic thinking, and away from a reactive “managed emergency”-style system that relies on private operators. Even in the middle of the housing crisis solutions exist. We then have to accept that providing asylum and accommodation is a positive and important part of being a modern democracy that respects human rights. An average of 2,290 people per year have claimed asylum in Ireland over the last 10 years. This is an entirely manageable number, but the majority have no means to pay for accommodation or family to rely on. Around 61,100 people have been accommodated in direct provision since 2000. If direct provision ends, something has to take its place. Responsibility should be shifted away from the Department of Justice. -

Public Health Law During the COVID-19 Pandemic in Ireland
Public Health Law During the COVID-19 Pandemic in Ireland Editors: Alan Eustace, Sarah Hamill, Andrea Mulligan A Public Policy Report of the COVID-19 LEGAL OBSERVATORY School of Law, Trinity College Dublin https://www.tcd.ie/law/tricon/covidobservatory/ Harnessing Trinity’s Collective Expertise for the Greater Good 3 August 2021 i ABOUT THE COVID-19 LEGAL OBSERVATORY Harnessing Trinity’s Collective Expertise for the Greater Good COVID-19 presents an unprecedented public health crisis. New laws were introduced at a rapid pace on the basis of compelling public health and economic concerns. Universities play a vital role in ensuring that laws are effective but also that rights and fundamental freedoms are protected insofar as possible, even in emergency circumstances. To address this, the COVID-19 Law and Human Rights Observatory1 of Trinity College Dublin engages in research across the full range of Ireland’s legal response to COVID-19. Academics in the Observatory the work with research assistants to identify, aggregate, contextualise, explain, and analyse the legal components of Ireland’s COVID-19 response. We aim both to inform the public and to provoke public debate. The Observatory’s Blog2 publishes academic commentary on Ireland’s legal response to COVID-19 as it evolves. The Observatory also provides an unofficial consolidated version of Ireland’s regulatory response to COVID-19, as well as a range of official guidance documents. This is the fourth public policy report of the Observatory. The first report, COVID-19: Public Policy Report on Supporting Individuals examined how public policy could support individuals against the backdrop of COVID-19;3 the second report, Ireland’s Emergency Powers During COVID-19 was completed on behalf of the Irish Human Rights and Equality Commission and explored how Ireland deployed emergency powers during the pandemic;4 the third report, A Right to Disconnect: Irish and European Legal Perspectives, explored 1 https://www.tcd.ie/law/tricon/covidobservatory/index.php. -

1 National Public Health Emergency Team – COVID-19 Meeting Note
National Public Health Emergency Team – COVID-19 Meeting Note – Standing Meeting Date and Time Thursday 28th May 2020, (Meeting 33) at 10:00am Location Department of Health, Miesian Plaza, Dublin 2 Chair Dr Tony Holohan, Chief Medical Officer, DOH Dr Kevin Kelleher, Assistant National Director, Public Health, HSE Mr Liam Woods, National Director, Acute Operations, HSE Dr Darina O’Flanagan, Special Advisor to the NPHET Prof Philip Nolan, President, National University of Ireland, Maynooth and Chair of the Irish Epidemiological Modelling Advisory Group (IEMAG) Dr Lorraine Doherty, National Clinical Director Health Protection, HSE Dr Cillian de Gascun, Laboratory Director, NVRL and Expert Advisory Group (EAG) Chair Mr David Leach, Communications, HSE Dr Mary Favier, President, Irish College of General Practitioners (ICGP) Mr Phelim Quinn, Chief Executive Officer, HIQA Dr Michael Power, Consultant in Anaesthetics / Intensive Care Medicine, Beaumont Hospital Dr Máirín Ryan, Deputy Chief Executive and Director of Health Technology Assessment, HIQA Dr Ronan Glynn, Deputy Chief Medical Officer, DOH Members via Dr Alan Smith, Deputy Chief Medical Officer, DOH videoconference Dr Eibhlin Connolly, Deputy Chief Medical Officer, DOH Dr Siobhan O’Sullivan, Chief Bioethics Officer, DOH Ms Tracey Conroy, Assistant Secretary, Acute Hospitals Policy Division, DOH Mr Fergal Goodman, Assistant Secretary, Primary Care Division, DOH Mr Colm Desmond, Assistant Secretary, Corporate Legislation, Mental Health, Drugs Policy and Food Safety Division, DOH Mr Paul -
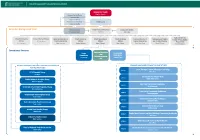
Executive Management Team Operational Services
Executive Management Team and Operational Services Minister for Health Stephen Donnelly Board Committees Audit and Risk People and Culture Performance and Delivery HSE Board Safety and Quality Executive Management Team Internal Audit Chief Executive Officer Corporate Affairs Dr Geraldine Smith Paul Reid John Kelly National Director Chief Information Chief Clinical Officer National Director of Chief Financial Chief Operations Chief Strategy National Director of National Lead Testing COVID-19 Vaccination Officer Dr Colm Henry Communications Officer Officer Officer Human Resources and Contact Tracing Programme Fran Thompson Mark Brennock Stephen Mulvany Anne O’Connor Dean Sullivan Anne Marie Hoey Niamh O’Beirne Damien McCallion Operational Services National Schemes Acute Community and Operations Operations Reimbursements Liam Woods Yvonne O’Neill TBC community healthcare organisations chief officers hospital groups ceos Cavan, Donegal, Leitrim, Monaghan and Sligo area 1 RCSI Hospital Group John Hayes Ian Carter Community Healthcare West area 2 Dublin Midlands Hospital Group Breda Crehan Roche Trevor O’Callaghan Mid West Community Healthcare area 3 University of Limerick Hospitals Group Maria Bridgeman Prof Colette Cowan Cork, Kerry, Community Healthcare area 4 Michael Fitzgerald South/South West Hospital Group Gerry O’Dwyer South East Community Healthcare area 5 Kate Killeen White Saolta University Health Care Group Tony Canavan area 6 Community Healthcare East Martina Queally Ireland East Hospital Group Declan Lyons area 7 Dublin South, Kildare and West Wicklow Community Healthcare Ann O’Shea Children’s Health Ireland Eilish Hardiman area 8 Midlands Louth Community Healthcare Des O’Flynn Head of National Ambulance Service Dublin North City and County Community Healthcare area 9 Robert Morton Mellany McLoone. -

1 National Public Health Emergency Team – COVID-19 Meeting Note
National Public Health Emergency Team – COVID-19 Meeting Note Date and Time Sunday 4th October 2020, (Meeting 57) at 12:00pm Location Department of Health, Miesian Plaza, Dublin 2 Chair Dr Tony Holohan, Chief Medical Officer, DOH Dr Ronan Glynn, Deputy Chief Medical Officer, DOH Dr Kevin Kelleher, Assistant National Director, Public Health, HSE Prof Philip Nolan, President, National University of Ireland, Maynooth and Chair of the Irish Epidemiological Modelling Advisory Group (IEMAG) Dr Cillian de Gascun, Laboratory Director, NVRL and Expert Advisory Group (EAG) Chair Dr Máirín Ryan, Deputy Chief Executive and Director of HTA, HIQA Dr John Cuddihy, Interim Director, HSE HPSC Prof Colm Bergin, Consultant in Infectious Diseases, St James’s Hospital Dr Michael Power, Consultant in Anaesthetics / Intensive Care Medicine, Beaumont Hospital Dr Eibhlín Connolly, Deputy Chief Medical Officer, DOH Dr Mary Favier, Immediate past president of the ICGP, Covid-19 advisor Ms Tracey Conroy, Assistant Secretary, Acute Hospitals Policy Division, DOH Dr Siobhán O’Sullivan, Chief Bioethics Officer, DOH Dr Colette Bonner, Deputy Chief Medical Officer, DOH Mr Colm Desmond, Assistant Secretary, Corporate Legislation, Mental Health, Drugs Policy and Food Safety Division, DOH Members via Mr Phelim Quinn, Chief Executive Officer, HIQA videoconference Dr Darina O’Flanagan, Special Advisor to the NPHET Mr Fergal Goodman, Assistant Secretary, Primary Care Division, DOH Dr Breda Smyth, Public Health Specialist, HSE Mr Tom McGuinness, Assistant National Director -

High Level Task Force on COVID-19 Vaccination
High Level Task Force on COVID-19 Vaccination 8 February 2021 Meeting Updates, decisions and actions from meeting High Level Taskforce on COVID-19 Vaccination | 8 February 2021 Meeting High Level Task Force on COVID-19 Vaccination Monday 8 February 2021 14:00 Updates, decisions and actions arising from meeting 1. Attendees A. Members in attendance B. Additional attendees in support Prof Brian MacCraith, Task Force Chair ii. In Attendance Liz Canavan, Chair, SOG on COVID-19 Sean Bresnan, National Director of Procurement, HSE Fergal Goodman, Assistant Secretary, Health Dr Ronan Glynn, Deputy CMO, DOH Protection Division, DOH Dr Colm Henry, Chief Clinical Officer, HSE Deirdre Watters, Head of Communications, DOH Rachel Kenna, Chief Nursing Officer, DOH Elizabeth Headon, Programme Communications Barry Lowry, Chief Information Officer, OGCIO Dr Lucy Jessop, SRO WS2, Director, NIO, HSE Derek McCormack, Expert on Cold Chain Logistics Paul Flanagan, SRO WS3 Dermot Mulligan, Assistant Secretary, Innovation David Walsh, SRO WS4 and Investment Division, DETE Lorraine Nolan, Chief Executive, HPRA Dr John Cuddihy, SRO WS5 Dr Nuala O'Connor, ICGP Fran Thompson, SRO WS6 Dalton Philips, Chief Executive Officer, DAA David Leach, SRO WS7 Paul Quinn, Government CPO and CEO, OGP Andrew Byrne, Immunisation and Infectious Diseases Policy, DOH Paul Reid, Chief Executive Officer, HSE Deirdre McNamara, General Manager, Quality & Patient Safety, Acute Hospitals Division, HSE Martin Shanahan, Chief Executive Officer, IDA Minister Stephen Donnelly, Minister for Health Derek Tierney, Programme Director iii. In partial attendance Additional attendees in support Frances McNamara, Senior Operations and Corporate, HSE i. Task Force Secretariat iv. Programme support Kate Waterhouse, Task Force Secretariat Yvonne Mowlds (PWC), Programme Office Apologies: Prof Karina Butler, Chair, NIAC 2 High Level Taskforce on COVID-19 Vaccination | 8 February 2021 Meeting 2. -

Link to CAO Annual Report 2020
CHIEF ACADEMIC OFFICERS IRELAND ANNUAL REPORT 2020 C A O G R O U P | A N N U A L R E P O R T 0 1 CHIEF ACADEMIC OFFICERS Professor Paul Burke, MD FRCSI, Associate Professor, Vice Dean (Health Sciences) - University of Limerick, Adjunct Professor of Surgery, UL GEMS, Chief Academic Officer - UL Hospital Group Professor Arnie Hill, Chair of Surgery - RCSI. Chief Academic Officer, RCSI Hospitals Professor Joseph Keane, Consultant Respiratory Physician, Head of Clinical Medicine – St James’s Hospital, Dublin Midlands Hospital Group HSE Professor Timothy Lynch, Consultant Neurologist - Mater Misericordiae Hospital, VP Health Affairs - UCD, Chief Academic Officer –Ireland East Hospital Group Professor Anthony O'Regan, Consultant Physician - Galway University Hospital, Chief Academic Officer –Saolta University Health Care Group Professor Owen Smith, UCD Professor of Paediatric and Adolescent Medicine, Consultant Paediatric Haematologist at Children’s Health Ireland at Crumlin, Chief Academic Lead to the Children's Hospital Group Professor Helen Whelton, Head of College of Medicine and Health - University College Cork, Chief Academic Officer –HSE South, South West Hospital Group C A O G R O U P | A N N U A L R E P O R T 0 2 A LETTER FROM THE CAO GROUP As Chief Academic Officers (CAOs) we act as a link between our hospital groups and universities – ensuring innovation, research and teaching remain at the forefront of Irish healthcare. As COVID-19 made itself known throughout Ireland in 2020 we begun to harness our resources to focus on areas that needed urgent attention. In the initial stages of the COVID-19 pandemic the CAO’s worked with their partner universities, hospital groups and industry to assist with laboratory diagnostics. -

August 2020 College of Medicine and Health, UCC
CoMH eNEWS Issue 37 | August 2020 College of Medicine and Health, UCC Navigate stories Welcome to our latest CoMH online newsletter New microbiome research of practical value to patients Congratulations to Virtual Conferrings » Graduates of our Medical and Summer Conferrings Infant & Dentistry Researchers awarded ¤500,000 in HRB funding Two awards for ‘Outstanding Junior Scientist’ Dr Erin Harris MSc in Human Anatomy open on PAC Research » HRB Funding » CRF-C to play lead role in WHO COVID-19 Solidarity Trial Other news CoMH eNews is intended for circulation among staff and students of the College of Medicine and Health, UCC. Extracts from CoMH eNews should not be published without the permission of the editor. Awards » Courses » Coronavirus Response » For info and submissions email: [email protected] Start reading CoMH eNews CoMH eNEWS Message from Head of College Issue 37 | August 2020 Our researchers continue to be extremely partner of APC, discovers, develops and ¤16m study ‘SOPHIA’ to better understand active in the race to understand and markets probiotics (live bacteria), which can obesity and optimise future treatments. mitigate COVID-19. The director of CRF-C, improve gut health in animals and humans. professor Joe Eustace was nominated by the CoMH researchers were successful in 3 Minister of Health to be the National Chief I’d like to congratulate Professor Chris Lynch, separate H2020 funding initiatives. APC investigator for the WHO Solidarity Clinical professor and consultant in Restorative Microbiome Ireland researchers Dr Gerard Trial in Ireland, with the Department of Health Dentistry who is a recipient of Ireland’s Clarke and Professor John F. -

High Level Task Force on COVID-19 Vaccination Monday 19 April 2021 14:00 Updates, Decisions and Actions Arising from Meeting 1
High Level Task Force on COVID-19 Vaccination 19 April 2021 Meeting Updates, decisions and actions from meeting High Level Taskforce on COVID-19 Vaccination | 19 April 2021 Meeting High Level Task Force on COVID-19 Vaccination Monday 19 April 2021 14:00 Updates, decisions and actions arising from meeting 1. Attendees A. Members in attendance B. Additional attendees in support Prof Brian MacCraith, Task Force Chair Kate Waterhouse, Task Force Secretariat Prof Karina Butler, Chair, NIAC Sean Bresnan, National Director of Procurement, HSE Liz Canavan, Chair, SOG on COVID-19 Dr Lorraine Doherty, Clinical Director Health Protection, HSE Fergal Goodman, Assistant Secretary, Health Dr Ronan Glynn, Acting CMO, DOH Protection Division, DOH Dr Colm Henry, Chief Clinical Officer, HSE Gerry O’Brien, Director, Health Protection, DOH Rachel Kenna, Chief Nursing Officer, DOH Deirdre Watters, Head of Communications, DOH Barry Lowry, Chief Information Officer, OGCIO Dr Lucy Jessop, SRO WS2, Director, NIO Derek McCormack, Expert on Cold Chain Logistics David Walsh, SRO WS4 Dermot Mulligan, Assistant Secretary, Innovation Dr John Cuddihy, SRO WS5 and Investment Division, DETE Dr Nuala O'Connor, ICGP Fran Thompson, SRO WS6 Lorraine Nolan, Chief Executive, HPRA David Leach, SRO WS7 Dalton Philips, Chief Executive Officer, DAA Eileen Hearne, Government Information Service Paul Quinn, Government CPO and CEO, OGP Damien McCallion, National Director, HSE Paul Reid, Chief Executive Officer, HSE Deirdre McNamara, General Manager, Quality & Patient Safety, Acute Hospitals Division, HSE Martin Shanahan, Chief Executive Officer, IDA Minister Stephen Donnelly TD, Minister for Health Derek Tierney, Programme Director Michael McDaid (PWC), Programme Office Yvonne Mowlds (PWC), Programme Office Fiona Smith (PWC), Programme Office 2 High Level Taskforce on COVID-19 Vaccination | 19 April 2021 Meeting 2.