High Level Task Force on COVID-19 Vaccination Monday 19 April 2021 14:00 Updates, Decisions and Actions Arising from Meeting 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
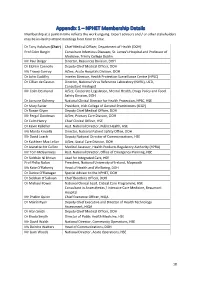
Appendix 1 – NPHET Membership Details Membership at a Point in Time Reflects the Work Ongoing
Appendix 1 – NPHET Membership Details Membership at a point in time reflects the work ongoing. Expert advisors and / or other stakeholders may be invited to attend meetings from time to time. Dr Tony Holohan (Chair) Chief Medical Officer, Department of Health (DOH) Prof Colm Bergin Consultant Infectious Diseases, St. James’s Hospital and Professor of Medicine, Trinity College Dublin Mr Paul Bolger Director, Resources Division, DOH Dr Eibhlin Connolly Deputy Chief Medical Officer, DOH Ms Tracey Conroy A/Sec, Acute Hospitals Division, DOH Dr John Cuddihy Interim Director, Health Protection Surveillance Centre (HPSC) Dr Cillian de Gascun Director, National Virus Reference Laboratory (NVRL), UCD, Consultant Virologist Mr Colm Desmond A/Sec, Corporate Legislation, Mental Health, Drugs Policy and Food Safety Division, DOH Dr Lorraine Doherty National Clinical Director for Health Protection, HPSC, HSE Dr Mary Favier President, Irish College of General Practitioners (ICGP) Dr Ronan Glynn Deputy Chief Medical Officer, DOH Mr Fergal Goodman A/Sec, Primary Care Division, DOH Dr Colm Henry Chief Clinical Officer, HSE Dr Kevin Kelleher Asst. National Director, Public Health, HSE Ms Marita Kinsella Director, National Patient Safety Office, DOH Mr David Leach Deputy National Director of Communications, HSE Dr Kathleen Mac Lellan A/Sec, Social Care Division, DOH Dr Jeanette Mc Callion Medical Assessor, Health Products Regulatory Authority (HPRA) Mr Tom McGuinness Asst. National Director, Office of Emergency Planning, HSE Dr Siobhán Ní Bhrian Lead for -

Dáil Éireann
Vol. 1006 Wednesday, No. 7 12 May 2021 DÍOSPÓIREACHTAÍ PARLAIMINTE PARLIAMENTARY DEBATES DÁIL ÉIREANN TUAIRISC OIFIGIÚIL—Neamhcheartaithe (OFFICIAL REPORT—Unrevised) Insert Date Here 12/05/2021A00100Ábhair Shaincheisteanna Tráthúla - Topical Issue Matters 884 12/05/2021A00175Saincheisteanna Tráthúla - Topical Issue Debate 885 12/05/2021A00200Digital Hubs ����������������������������������������������������������������������������������������������������������������������������������������������������������885 12/05/2021B00350Hospital Waiting Lists 887 12/05/2021C00400Special Educational Needs 891 12/05/2021E00300Harbours and Piers 894 12/05/2021F00600Companies (Protection of Employees’ Rights in Liquidations) Bill 2021: Second Stage [Private Members] 897 12/05/2021S00500Ceisteanna ó Cheannairí - Leaders’ Questions 925 12/05/2021W00500Ceisteanna ar Reachtaíocht a Gealladh - Questions on Promised Legislation 935 12/05/2021AA00800Pensions (Amendment) (Transparency in Charges) Bill 2021: First Stage 945 12/05/2021AA01700Health (Regulation of Termination of Pregnancy) (Foetal Pain Relief) Bill 2021: First Stage 946 12/05/2021BB00900Ministerial Rota for Parliamentary Questions: Motion -

What Are the Alternatives to Our Broken Direct Provision System?
Position Paper What are the alternatives to our broken Direct Provision system? February 2019 Direct provision is already a chapter in Ireland’s dark history of institutional living. More of the same fails us all. Direct provision is broken, condemned by politicians across the political spectrum, international organisations and, most importantly, by residents themselves. Geoffrey Shannon, the special rapporteur on child protection, called for it to be abolished in his recent annual report. Its failures have been vividly demonstrated through Melatu Uche Okorie’s book Hostel People and the TV show Taken Down. So how do we end direct provision, what is the alternative and how do we get there? Minister of State for Justice David Stanton repeatedly says he has not heard of a better system. These are some ideas. In short, we need to shift to long-term, strategic thinking, and away from a reactive “managed emergency”-style system that relies on private operators. Even in the middle of the housing crisis solutions exist. We then have to accept that providing asylum and accommodation is a positive and important part of being a modern democracy that respects human rights. An average of 2,290 people per year have claimed asylum in Ireland over the last 10 years. This is an entirely manageable number, but the majority have no means to pay for accommodation or family to rely on. Around 61,100 people have been accommodated in direct provision since 2000. If direct provision ends, something has to take its place. Responsibility should be shifted away from the Department of Justice. -

Public Health Law During the COVID-19 Pandemic in Ireland
Public Health Law During the COVID-19 Pandemic in Ireland Editors: Alan Eustace, Sarah Hamill, Andrea Mulligan A Public Policy Report of the COVID-19 LEGAL OBSERVATORY School of Law, Trinity College Dublin https://www.tcd.ie/law/tricon/covidobservatory/ Harnessing Trinity’s Collective Expertise for the Greater Good 3 August 2021 i ABOUT THE COVID-19 LEGAL OBSERVATORY Harnessing Trinity’s Collective Expertise for the Greater Good COVID-19 presents an unprecedented public health crisis. New laws were introduced at a rapid pace on the basis of compelling public health and economic concerns. Universities play a vital role in ensuring that laws are effective but also that rights and fundamental freedoms are protected insofar as possible, even in emergency circumstances. To address this, the COVID-19 Law and Human Rights Observatory1 of Trinity College Dublin engages in research across the full range of Ireland’s legal response to COVID-19. Academics in the Observatory the work with research assistants to identify, aggregate, contextualise, explain, and analyse the legal components of Ireland’s COVID-19 response. We aim both to inform the public and to provoke public debate. The Observatory’s Blog2 publishes academic commentary on Ireland’s legal response to COVID-19 as it evolves. The Observatory also provides an unofficial consolidated version of Ireland’s regulatory response to COVID-19, as well as a range of official guidance documents. This is the fourth public policy report of the Observatory. The first report, COVID-19: Public Policy Report on Supporting Individuals examined how public policy could support individuals against the backdrop of COVID-19;3 the second report, Ireland’s Emergency Powers During COVID-19 was completed on behalf of the Irish Human Rights and Equality Commission and explored how Ireland deployed emergency powers during the pandemic;4 the third report, A Right to Disconnect: Irish and European Legal Perspectives, explored 1 https://www.tcd.ie/law/tricon/covidobservatory/index.php. -

1 National Public Health Emergency Team – COVID-19 Meeting Note
National Public Health Emergency Team – COVID-19 Meeting Note Date and Time Sunday 4th October 2020, (Meeting 57) at 12:00pm Location Department of Health, Miesian Plaza, Dublin 2 Chair Dr Tony Holohan, Chief Medical Officer, DOH Dr Ronan Glynn, Deputy Chief Medical Officer, DOH Dr Kevin Kelleher, Assistant National Director, Public Health, HSE Prof Philip Nolan, President, National University of Ireland, Maynooth and Chair of the Irish Epidemiological Modelling Advisory Group (IEMAG) Dr Cillian de Gascun, Laboratory Director, NVRL and Expert Advisory Group (EAG) Chair Dr Máirín Ryan, Deputy Chief Executive and Director of HTA, HIQA Dr John Cuddihy, Interim Director, HSE HPSC Prof Colm Bergin, Consultant in Infectious Diseases, St James’s Hospital Dr Michael Power, Consultant in Anaesthetics / Intensive Care Medicine, Beaumont Hospital Dr Eibhlín Connolly, Deputy Chief Medical Officer, DOH Dr Mary Favier, Immediate past president of the ICGP, Covid-19 advisor Ms Tracey Conroy, Assistant Secretary, Acute Hospitals Policy Division, DOH Dr Siobhán O’Sullivan, Chief Bioethics Officer, DOH Dr Colette Bonner, Deputy Chief Medical Officer, DOH Mr Colm Desmond, Assistant Secretary, Corporate Legislation, Mental Health, Drugs Policy and Food Safety Division, DOH Members via Mr Phelim Quinn, Chief Executive Officer, HIQA videoconference Dr Darina O’Flanagan, Special Advisor to the NPHET Mr Fergal Goodman, Assistant Secretary, Primary Care Division, DOH Dr Breda Smyth, Public Health Specialist, HSE Mr Tom McGuinness, Assistant National Director -

Dáil Éireann
DÁIL ÉIREANN AN COMHCHOISTE UM SHLÁINTE JOINT COMMITTEE ON HEALTH Dé Máirt, 13 Aibreán 2021 Tuesday, 13 April 2021 Tháinig an Comhchoiste le chéile ag 12.30 p.m. The Joint Committee met at 12.30 p.m. Comhaltaí a bhí i láthair/Members present: Teachtaí Dála/Deputies Seanadóirí/Senators Colm Burke, Frances Black, Cathal Crowe, Lorraine Clifford-Lee, David Cullinane, Martin Conway, Bernard J. Durkan, Annie Hoey, Neasa Hourigan, Seán Kyne. Gino Kenny, John Lahart, Róisín Shortall. I láthair/In attendance: Deputy Peadar Tóibín. Teachta/Deputy Seán Crowe sa Chathaoir/in the Chair. 1 JH Business of Joint Committee Chairman: There are no apologies. We have our full membership present. We have one item of committee business to be addressed before I call our witness, Dr. Ronan Glynn, to pres- ent to us. I take it that the draft minutes of our private and public meetings of last Thursday, 8 April, are agreed and that there are no matters arising. Is that agreed? Agreed. Deputy David Cullinane: May I raise an issue, please? Chairman: Please, go ahead. Deputy David Cullinane: I wish to raise the issue of the decision of the national immuni- sation advisory committee, NIAC, yesterday regarding the AstraZeneca vaccine. Obviously, this is a matter for NIAC in terms of the public health measure and putting safety first. I have no problem with that. It is more the implications of the decision. We need to hear from the HSE and NIAC next week that they have a plan to mitigate this decision because we do not want this to interrupt in any way the roll-out. -

Statement from the National Public Health Emergency Team - Thursday 14 January
Statement from the National Public Health Emergency Team - Thursday 14 January From Department of Health Published on 14 January 2021 Last updated on 14 January 2021 • 1. Today's update • 2. Breakdown of today's cases • 3. View slides from today's press conference Today's update The Health Protection Surveillance Centre has today been notified of 28 additional deaths related to COVID-19. 26 of these deaths occurred in January 2021. The date of death for 2 of these reported deaths remains under investigation. There has been a total of 2,488 COVID-19 related deaths in Ireland. As of midnight Wednesday 13 January, the HPSC has been notified of 3,955 confirmed cases of COVID-19. There has now been a total of 163,057* confirmed cases of COVID-19 in Ireland. (*Validation of data at the HPSC has resulted in the denotification of 42 confirmed cases. The figure of 163,057 confirmed cases reflects this.) Of the cases notified today: • 1,826 are men and 2,115 are women • 54% are under 45 years of age • the median age is 42 years old • 1,210 are in Dublin, 456 in Cork, 235 in Louth, 221 in Meath, 218 in Limerick, and the remaining 1,615 cases are spread across all other counties As of 2pm today, 1,789 COVID-19 patients are hospitalised, of which 169 are in ICU. There have been 154 additional hospitalisations in the past 24 hours. Dr Tony Holohan, Chief Medical Officer, Department of Health said: "Today we are giving some more information on the 208 people who have been reported to have sadly died from COVID-19 so far this month. -

1 National Public Health Emergency Team – COVID-19 Meeting Note
National Public Health Emergency Team – COVID-19 Meeting Note – Standing meeting Date and Time Wednesday 25th November 2020, (Meeting 65) at 11:00am Location Department of Health, Miesian Plaza, Dublin 2 Chair Dr Tony Holohan, Chief Medical Officer, DOH Dr Ronan Glynn, Deputy Chief Medical Officer, DOH Dr Kevin Kelleher, Assistant National Director, Public Health, HSE Prof Philip Nolan, President, National University of Ireland, Maynooth and Chair of the Irish Epidemiological Modelling Advisory Group (IEMAG) Dr Cillian de Gascun, Laboratory Director, NVRL and Expert Advisory Group (EAG) Chair Dr Máirín Ryan, Deputy Chief Executive and Director of HTA, HIQA Dr John Cuddihy, Interim Director, HSE HPSC Prof Colm Bergin, Consultant in Infectious Diseases, St James’s Hospital Dr Siobhán Ní Bhriain, Lead for Integrated Care, HSE Dr Eibhlín Connolly, Deputy Chief Medical Officer, DOH Dr Mary Favier, Immediate past president of the ICGP, Covid-19 advisor Ms Tracey Conroy, Assistant Secretary, Acute Hospitals Policy Division, DOH Dr Siobhán O’Sullivan, Chief Bioethics Officer, DOH Dr Michael Power, Consultant in Anaesthetics / Intensive Care Medicine, Beaumont Hospital Members via Dr Lorraine Doherty, National Clinical Director Health Protection, HSE videoconference Ms Yvonne O’Neill, National Director, Community Operations, HSE Mr Phelim Quinn, Chief Executive Officer, HIQA Dr Matthew Robinson, Specialist Registrar in Public Health, DOH Mr Colm Desmond, Assistant Secretary, Corporate Legislation, Mental Health, Drugs Policy and Food Safety Division, -

Downloaded from the WHO Global Observatory on Health R&D
Published by: The Department of Health Hawkins House Hawkins Street Dublin 2 D02 VW90 Ireland Tel: +353 (1) 6354000 www.health.gov.ie October 2017 ©Department of Health, October 2017 Citation text Department of Health (2017). Ireland’s National Action Plan on Antimicrobial Resistance 2017-2020. (iNAP) Available at http://health.gov.ie/national-patient-safety-office/patient-safety-surveillance/antimicrobial- resistance-amr/ In text citation (Department of Health 2017) A post-antibiotic era means, “in effect, an end to modern medicine as we know it. – Dr Margaret Chan, Former WHO Director General - March 2012 ” Ireland’s National Action Plan on Antimicrobial Resistance 2017 – 2020 Table of contents Ministerial Foreword 6 Departmental Foreword 7 Key concepts and definitions 8 1. Introduction 10 2. Situational analyses and assessment – Human Health 15 3. Situational analyses and assessment – Animal Health 38 4. Situational analyses and assessment – Environmental Health 50 5. Governance and responsibilities for implementation of Ireland’s National Action Plan on Antimicrobial Resistance 2017-2020 53 6. Ireland’s 3-Year Plan - Priority Strategic Interventions and Activities 58 Strategic Objective 1: Improve awareness and knowledge of antimicrobial resistance 65 Strategic Objective 2: Enhance surveillance of antibiotic resistance and antibiotic use 71 Strategic Objective 3: Reduce the spread of infection and disease 77 Strategic Objective 4: Optimise the use of antibiotics in human and animal health 83 Strategic Objective 5: Promote research and sustainable investment in new medicines, diagnostic tools, vaccines and other interventions 89 Appendix 1: National Interdepartmental Antimicrobial Resistance Consultative Committee 95 Appendix 2: List of key stakeholders 97 Appendix 3: HPSC surveillance of key pathogens 101 Glossary and abbreviations 103 Page 3 Ireland’s National Action Plan on Antimicrobial Resistance 2017 – 2020 List of Figures Page Figure 1: Key groups, agencies and health professionals. -

Twenty Fourth Plenary Meeting
NORTH SOUTH MINISTERIAL COUNCIL TWENTY-FOURTH PLENARY MEETING DUBLIN CASTLE 31 JULY 2020 JOINT COMMUNIQUÉ 1. The twenty-fourth Plenary meeting of the North South Ministerial Council (NSMC) was held at Dublin Castle on 31 July 2020. 2. The Irish Government was led by the Taoiseach, Micheál Martin TD, who chaired the meeting. The Northern Ireland Executive was led by the First Minister, the Rt. Hon. Arlene Foster MLA, and the deputy First Minister, Michelle O’Neill MLA. A full list of the members of both delegations is attached as an Annex. 3. Ministers welcomed the resumption of meetings of the Council. The meeting provided the new Irish Government and the new Northern Ireland Executive with the opportunity to meet formally for the first time and exchange views on a wide range of issues of mutual interest and concern. WORK OF THE NORTH SOUTH MINISTERIAL COUNCIL 4. Ministers received a report from the Joint Secretaries on the work of the NSMC since 2016, including work undertaken across the NSMC sectors. They noted that three meetings of the Council, including one Institutional meeting, had taken place since the last Plenary meeting in November 2016. 5. The Council noted that the work of the North South Bodies has continued to make a significant contribution to communities, society and the economy in both jurisdictions, and expressed appreciation to the Boards and staff of these bodies for their work since 2016. The Council also welcomed the mutually beneficial 1 cooperation that has been taken forward between Ministers and their Departments across the Areas of Cooperation. -

IASW Letter to Dr Ryan, HIQA Re. The
Dr Mairin Ryan Deputy Chief executive HIQA George’s Court, George’s Lane, Dublin 7 D07 E98Y 8th October 2020 Re. The absence of social work clinical expertise on the EAG to NPHET Dr Ryan, I write on behalf of the Irish Association of Social Workers (IASW) with serious concerns about the omission of social work from the membership of the HIQA Expert Advisory Group to NPHET. The absence of social work clinical expertise on the EAG to NPHET reflects a consistent pattern in omitting the vital expertise of registered health and social care professionals from the national response to Covid-19. To rectify this oversight, I propose that IASW member Dr Sarah Donnelly, Assistant Professor in the School of Social Work, UCD, is invited to join the HIQA EAG group at the earliest opportunity. Sarah is author of the 'Falling through the Cracks' report, is an expert in the field of adult safeguarding and can ably represent the broader expertise/ knowledge base of the social work profession in nursing homes and across Irish health and social care services. Please note, the Expert Panel on Nursing Homes Report identified social work as an essential service and given social work's role in health and social care delivery across the lifespan, our knowledge base is vital to the work of NPHET. In Northern Ireland the social work profession, led by Chief Social Worker Sean Holland, has held a leadership role throughout the region’s response to the pandemic. The value placed on the knowledge base and expertise of social work is evident there in the profession’s representation on key consultation bodies, expert advisory groups etc. -

VIRTUAL NATIONAL HEALTHCARE OUTCOMES CONFERENCE 13 April 2021
NATIONAL HEALTHCARE OUTCOMES CONFERENCE UNIVERSITY OF MEDICINE AND HEALTH SCIENCES Healthcare Outcomes Research Centre VIRTUAL NATIONAL HEALTHCARE OUTCOMES CONFERENCE 13 April 2021 Sustaining Healthcare in a COVID-19 World CPD Accreditation: 3 Credits 1 NATIONAL HEALTHCARE OUTCOMES CONFERENCE WELCOME On behalf of RCSI and the Healthcare Outcomes Research Centre, it gives us great pleasure to welcome you to the annual National Healthcare Outcomes Conference 2021. The Covid-19 pandemic has had dramatic consequences for healthcare delivery, for the global economy, and for the health and welfare of countless individuals. To protect both the population and the capacity of healthcare services, it was necessary to introduce many new restrictions to our lives and the way that healthcare is delivered. Suddenly, a “new” cohort of Covid-19 infected patients required intensive healthcare intervention, taking priority over less acute patient groups. Treatment waiting times for some non-Covid patients increased while, other patients have been offered new ways of receiving their treatment. The urgent need for change in healthcare delivery has been and remains extremely challenging. However, it has also provided opportunities to explore and test new solutions. The Covid vaccination roll-out offers some optimism, but it is clear that healthcare systems must learn and adapt. Many of the changes that have been made during the last year may inspire further developments towards a more sustainable model for healthcare delivery. More than a year into the pandemic, it is therefore timely for this conference to reflect on what the future of healthcare might look like. The conference is being delivered virtually in two sessions.