Senior Thesis.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
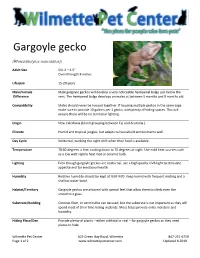
Gargoyle Gecko
Gargoyle gecko (Rhacodactylus auriculatus)) Adult Size SVL 4 – 4.5” Overall length 8 inches Lifespan 15-20 years Male/Female Male gargoyle geckos will develop a very noticeable hemipenal bulge just below the Difference vent. The hemipenal bulge develops on males at between 5 months and 9 months old. Compatibility Males should never be housed together. If housing multiple geckos in the same cage make sure to provide 10 gallons per 1 gecko, with plenty of hiding spaces. This will ensure there will be no territorial fighting. Origin New Caledonia (Island grouping between Fiji and Australia.) Climate Humid and tropical jungles, but adapts to household environments well. Day Cycle Nocturnal, working the night shift when their food is available. Temperature 78-82 degrees is fine, cooling down to 70 degrees at night. Use mild heat sources such as a low watt reptile heat mat or ceramic bulb. Lighting Even though gargoyle geckos are nocturnal, use a high quality UVA light to stimulate appetite and for emotional health. Humidity Relative humidity should be kept at %50-%70. Keep humid with frequent misting and a shallow water bowl. Habitat/Territory Gargoyle geckos are arboreal with special feet that allow them to climb even the smoothest glass. Substrate/Bedding Coconut fiber, or vermiculite can be used, but the substrate is not important as they will spend most of their time hiding in plants. Moss helps provide extra moisture and humidity. Hiding Place/Den Provide plenty of plants – either artificial or real – for gargoyle geckos as they need places to hide. Wilmette Pet Center 625 Green Bay Road, Wilmette 847-251-6750 Page 1 of 2 www.wilmettepetcenter.com Updated 4.2019 Cage Type Ten gallon aquariums or critter cages with screen tops work well for gargoyle geckos. -

Crested Gecko by Catherine Love, DVM Updated 2021
Crested Gecko By Catherine Love, DVM Updated 2021 Natural History Rhacodactylus ciliatus, more recently re-classified as Correlophus ciliatus, is a species of arboreal lizard native to New Caledonia. Until 1994, crested geckos were thought to be extinct in the wild. Their population was re-discovered, and although export is no longer allowed, this species has thrived in captivity and is readily available in the US pet trade. Cresties are crepuscular (active at dawn and dusk), and spend most of their day in shrubs or in trees. They get their name from the eyelash-like appendages that form the crests above their eyes. Unlike most geckos that are carnivorous, these animals are omnivorous, and primarily frugivores (fruit eaters). One of the biggest threats to wild crested geckos is the introduced little fire ant that not only swarms the geckos in large numbers, but also competes for arthropod prey. Crested geckos are considered “vulnerable” by the IUCN. Characteristics and Behavior As with most geckos, cresties do not have eyelids, so they keep their eyes moist by licking them. Cresties are also able to climb vertical surfaces using tiny hairs on their feet called setae. Their tails are partially prehensile, though not to the same extent as a chameleon, and they possess tail autotomy (they can drop their tails). Unlike leopard geckos, when a crestie drops their tail, it doesn’t grow back. However, they don’t store fat in their tails so it is generally not as detrimental for a crestie to drop their tail, and the majority of wild cresties end up doing so. -

RVC Exotics Service CRESTED GECKO CARE
RVC Exotics Service Royal Veterinary College Royal College Street London NW1 0TU T: 0207 554 3528 F: 0207 388 8124 www.rvc.ac.uk/BSAH CRESTED GECKO CARE The Crested gecko (Rhacodactylus ciliatus) originates from the islands of New Caledonia where the species was believed to be extinct until 1994 when it was subsequently rediscovered. In its natural environment, it can be found resting in rainforests, sleeping in trunk hollows or leaf litter, and only becomes active at night. Similar to other geckos, it can shed its tail as a defence mechanism (autotomy), but unlike others it lacks an ability to regenerate the tail, so care should be taken while handling. Geckos may live 10-15 years if looked after correctly. HOUSING • As large a vivarium as possible should be provided to enable room for exercise, and a thermal gradient to be created along the length of the tank (hot to cold). Wooden or fibreglass vivaria are ideal as this provides the lizard with some visual security and ventilation can be provided at lizard level. • Good ventilation is required and additional ventilation holes may need to be created. • Hides are required to provide some security. Artificial plants, cardboard boxes, plant pots, logs or commercially available hides can be used. They should be placed both at the warm and cooler ends of the tank. One hide should contain damp moss, kitchen towel or vermiculite to provide a humid environment for shedding. • Substrates suitable for housing lizards include newspaper, Astroturf and some of the commercially available substrates. It is important that the substrates either cannot be eaten, or if they are, do not cause blockages as this can prove fatal. -

A New Locality for Correlophus Ciliatus and Rhacodactylus Leachianus (Sauria: Diplodactylidae) from Néhoué River, Northern New Caledonia
Herpetology Notes, volume 8: 553-555 (2015) (published online on 06 December 2015) A new locality for Correlophus ciliatus and Rhacodactylus leachianus (Sauria: Diplodactylidae) from Néhoué River, northern New Caledonia Mickaël Sanchez1, Jean-Jérôme Cassan2 and Thomas Duval3,* Giant geckos from New Caledonia (Pacific Ocean) We observed seven native gecko species: Bavayia are charismatic nocturnal lizards. This paraphyletic (aff.) cyclura (n=1), Bavayia (aff.) exsuccida (n=1), group is represented by three genera, Rhacodactylus, Correlophus ciliatus (n=1), Dierrogekko nehoueensis Correlophus and Mniarogekko, all endemic to Bauer, Jackman, Sadlier and Whitaker, 2006 (n=1), New Caledonia (Bauer et al., 2012). Rhacodactylus Eurydactylodes agricolae Henkel and Böhme, 2001 leachianus (Cuvier, 1829) is largely distributed on the (n=1), Mniarogekko jalu Bauer, Whitaker, Sadlier and Grande Terre including the Île des Pins and its satellite Jackman, 2012 (n=1) and Rhacodactylus leachianus islands, whereas Correlophus ciliatus Guichenot, 1866 (n=1). Also, the alien Hemidactylus frenatus Dumeril is mostly known in the southern part of the Grande and Bibron, 1836 (n=3) has been sighted. The occurrence Terre, the Île des Pins and its satellite islands (Bauer of C. ciliatus and R. leachianus (Fig. 2 and 3) represent et al., 2012). Here, we report a new locality for both new records for this site. Both gecko species were species in the north-western part of Grande Terre, along observed close to the ground, at a height of less than the Néhoué River (Fig. 1). 1.5 m. The Néhoué River is characterized by gallery forests It is the first time that R. leachianus is recorded in the growing on deep alluvial soils. -

Thermal Ecology and Habitat Utilization of Rhacodactylus Leachianus from New Caledonia (Squamata: Diplodactylidae)
SALAMANDRA 54(2)Thermal117–122 ecology and15 Mayhabitat 2018 utilizationISSN of 0036–3375 Rhacodactylus leachianus from New Caledonia Thermal ecology and habitat utilization of Rhacodactylus leachianus from New Caledonia (Squamata: Diplodactylidae) Peter Sound1, Friedrich Wilhelm Henkel2, Christian Langner3 & Alfred Seitz1 1) Zoologisches Institut, Abteilung V Populationsbiologie, Universität Mainz, Saarstr. 21, 55099 Mainz, Germany 2) Frielinger Weg 25a, 59174 Kamen, Germany 3) Alstätte 23, 48727 Billerbeck, Germany Corresponding author: Peter Sound, e-mail: [email protected] Received: 24 March 2015 Accepted: 6 February 2018 by Stefan Lötters Abstract. Five specimens (2 ♀, 3 ♂) of the gecko species Rhacodactylus leachianus were radio-tracked for 14 days on Île de Bayonnaise, New Caledonia. Using thermo-sensitive transmitters, specimens were mapped continuously every hour. All movements, behavioural expressions and core temperatures were manually recorded, while data loggers simultaneously recorded the temperatures of microhabitat structures. In an additional trial, all geckos captured on the island were marked with Passive Integrated Transponders (PITs): Twenty-two adults (13 ♀, 9 ♂) and one subadult were marked in total. The mapping of R. leachianus on this island was repeated during 2005, 2007 and 2013. Average core temperatures of 23.4°C (♀) and 24.1°C (♂) were recorded ranging of 18.2–32.0°C (♀) and 18.0–32.2°C (♂), respectively. No sex-dependent differences were noted, but a clear relation to the local air temperature was found. Gravid females showed higher core temperatures (by up to 3°C) during oviposition. The test for equality of variances showed differences between the core temperatures of both sexes and the air temperature, indicating the ability of both sexes for active thermoregulation. -

Reptile Vacation – Visiting Our Homelands by Chelsea Holden
Reptile Vacation – Visiting Our Homelands By Chelsea Holden It was the middle of winter, and the reptiles in the lab were getting restless. They were all tired of the snow and cold and wanted to go on a vacation. But where would they go? All of the reptiles thought that their homeland would be the best place to visit. The bearded dragons, Frieda and Freddie, were the first to speak up. “Let’s go to Australia!” said Freddie. “We can go and be warm with trees, rocks, and maybe some sand. The perfect spot would have to be Queensland. While basking in the sun, we’ll have it made!” “And if it gets too hot, we’ll cool off in the shade!” added Frieda. The Rankin’s dragons also agreed. “To Queensland! To Queensland!” they shouted with glee. The frilled dragons had other ideas. “Australia would be nice,” said Villain, “but we think it’d be better if we went somewhere warm and wetter. And we want to go hide in a tree!” The other frilled dragons could only agree. From across the room, there came a rustling sound. It was the sand boa, poking its head out of the ground! “Sand sounds lovely,” it hissed. “It’s the desert for me. African sands are the place to be! I’d burrow my way into the warm sands and take in the sights of the Great Pyramids.” The green tree python piped in: “You can keep your sand! It’s the trees for me! The rainforests of New Guinea are the place to be! I’ll lounge on the tree branches and hide in the canopies.” Overhearing the excitement, the Leachies joined in: “We know where some of the geckos would love to go. -

Gargoyle Gecko Reptile - a Cold-Blooded Vertebrate with Scaly Skin
Glossary Gargoyle Gecko Reptile - A cold-blooded vertebrate with scaly skin. The gargoyle gecko is a nocturnal, arboreal lizard Amphibian - A cold-blooded vertebrate that begins life as that makes for a great companion. They come in an aquatic animal and grows into a terrestrial adult with many colourations (morphs) and are best recognised lungs. for their big eyes. They are closely related to the Terrestrial - A ground dwelling animal. crested gecko. Males cannot be kept together as Arboreal - An animal that lives in trees. they are aggressive to one another but females can Diurnal - Awake in the day. Gargoyle be housed together. If keeping males and females Nocturnal- Awake during the night. together, it is best to have minimal 2 females to 1 UVB - Ultraviolet radiaton. Gecko male. They can live between 15 to 20 years. Colubrid - A family of snakes. Hybrid - Offspring from animals of different species. Morph - Colourations created due to genetics. Musk - Unpleasant odour released when an animal is stressed or feels threatened. Live plants are only available on special order If you require any further information, please ask our pet care advisors who will be very happy to help. Opening Times Monday - Saturday: 9am - 6pm Sunday: 9.30am - 4pm Chessington Garden Centre Leatherhead Road, Chessington, Surrey, KT9 2NG Care & Advice Sheet Tel: 01372 725 638 Email: [email protected] Web: www.chessingtongardencentre.co.uk Please recycle me once you’ve nished reading. Inspiration for your Home & Garden Substrate & Furnishings Food & Water Substrates that maintain high humidity are Gargoyle geckos are fruit eating geckos. The recommended such as humus bricks or coco fibre. -

Literature Cited in Lizards Natural History Database
Literature Cited in Lizards Natural History database Abdala, C. S., A. S. Quinteros, and R. E. Espinoza. 2008. Two new species of Liolaemus (Iguania: Liolaemidae) from the puna of northwestern Argentina. Herpetologica 64:458-471. Abdala, C. S., D. Baldo, R. A. Juárez, and R. E. Espinoza. 2016. The first parthenogenetic pleurodont Iguanian: a new all-female Liolaemus (Squamata: Liolaemidae) from western Argentina. Copeia 104:487-497. Abdala, C. S., J. C. Acosta, M. R. Cabrera, H. J. Villaviciencio, and J. Marinero. 2009. A new Andean Liolaemus of the L. montanus series (Squamata: Iguania: Liolaemidae) from western Argentina. South American Journal of Herpetology 4:91-102. Abdala, C. S., J. L. Acosta, J. C. Acosta, B. B. Alvarez, F. Arias, L. J. Avila, . S. M. Zalba. 2012. Categorización del estado de conservación de las lagartijas y anfisbenas de la República Argentina. Cuadernos de Herpetologia 26 (Suppl. 1):215-248. Abell, A. J. 1999. Male-female spacing patterns in the lizard, Sceloporus virgatus. Amphibia-Reptilia 20:185-194. Abts, M. L. 1987. Environment and variation in life history traits of the Chuckwalla, Sauromalus obesus. Ecological Monographs 57:215-232. Achaval, F., and A. Olmos. 2003. Anfibios y reptiles del Uruguay. Montevideo, Uruguay: Facultad de Ciencias. Achaval, F., and A. Olmos. 2007. Anfibio y reptiles del Uruguay, 3rd edn. Montevideo, Uruguay: Serie Fauna 1. Ackermann, T. 2006. Schreibers Glatkopfleguan Leiocephalus schreibersii. Munich, Germany: Natur und Tier. Ackley, J. W., P. J. Muelleman, R. E. Carter, R. W. Henderson, and R. Powell. 2009. A rapid assessment of herpetofaunal diversity in variously altered habitats on Dominica. -

Gargoyle Gecko (Rhacodactylus Auriculatus)
©ReptiFiles® — Where Better Reptile Care Begins Gargoyle Gecko (Rhacodactylus auriculatus) Difficulty: Easy Gargoyle geckos are medium-sized 6-8” long lizards that range from cream to dark brown in color with a variety of patterns depending on morph (a reptile that has been bred for a specific color and/or pattern). They have wide toes that give them the ability to climb up smooth surfaces, and a fringe that runs from above their eyes down their back. Gargoyle geckos are native to New Caledonia, a group of islands between Fiji and Australia. They are most commonly found on the islands of Grande Terre and the Isle of Pines. These islands feature a tropical rainforest habitat, where gargoyle geckos can be found among the trees and vines. Gargoyle geckos have a 15-20 year lifespan when cared for properly, and may live longer in some cases. Due to their manageable size, relatively simple care requirements, and tolerance toward humans, gargoyle geckos are popular first-time reptiles. Shopping List Front-opening 18” x 18” x 24” glass terrarium 40w white incandescent heat bulb 5.5” dome lamp with ceramic socket Plug-in lamp dimmer 5-6% T8 UVB bulb, 12” 14” T8 fluorescent hood, with reflector Plug-in light timer Infrared thermometer Pressure sprayer Digital thermometer/hygrometer 2-4” naturalistic substrate Live or artificial plants Vines Branches Magnetic feeding ledge Small gecko feeding cups Crested gecko diet powder Calcium supplement w/o D3 Housing Although gargoyle geckos are small, they still need an enclosure that is large enough to give them adequate opportunity to explore, hunt, and generally exercise natural behaviors. -

Interpet Catalogue / Page: 1 of 34 Aquatics
Interpet Catalogue / Page: 1 of 34 Aquatics Mini Encyclopedia of the Tropical Aquarium This mini encyclopedia is packed with sensible advice and practical guidance. The first part shows how to set up a freshwater tropical aquarium in the home. Part two explores further aspects of fishkeeping, including how to choose and use aquarium plants plus advice on feeding, breeding, health care and routine maintenance. Part three presents a wide-ranging selection of suitable fish, including details of their origin, ideal aquarium conditions, compatibility and breeding. Product Code: 0109 Pages: 208 Author: Sandford, Gina ISBN: 9781842861011 Binding: Paperback Size: 18.4cm x 15.6cm 1.8cm Mini Encyclopedia of The Marine Aquarium The traditional image of an encyclopedia as a large, heavy volume only occasionally taken down from a dusty shelf is blown out of the water by this new range of mini-encyclopedias. These are comprehensive, concentrated packages of up-to-date practical guidance on a variety of fishkeeping and water gardening themes. The core text is amplified and extended by panels, captions and annotations that accompany the photographs and graphic illustrations. The first part of each volume consists of a hands-on practical section that follows the step-by-step progress of a specific project. Accompanying text includes technical advice on the equipment required to complete each stage of the project. The second part features a wide range of species or varieties relevant to the subject. These profile sections are profusely illustrated with stunning colour photographs and are designed to inspire readers to expand their collections. By combining practical and reference sections, these mini encyclopedias represent the ideal compromise in a world where shelf space is limited but the thirst for knowledge increases every day. -

MAHS Care Sheet Master List *By Eric Roscoe Care Sheets Are Often An
MAHS Care Sheet Master List *By Eric Roscoe Care sheets are often an excellent starting point for learning more about the biology and husbandry of a given species, including their housing/enclosure requirements, temperament and handling, diet , and other aspects of care. MAHS itself has created many such care sheets for a wide range of reptiles, amphibians, and invertebrates we believe to have straightforward care requirements, and thus make suitable family and beginner’s to intermediate level pets. Some species with much more complex, difficult to meet, or impracticable care requirements than what can be adequately explained in a one page care sheet may be multiple pages. We can also provide additional links, resources, and information on these species we feel are reliable and trustworthy if requested. If you would like to request a copy of a care sheet for any of the species listed below, or have a suggestion for an animal you don’t see on our list, contact us to let us know! Unfortunately, for liability reasons, MAHS is unable to create or publish care sheets for medically significant venomous species. This includes species in the families Crotilidae, Viperidae, and Elapidae, as well as the Helodermatidae (the Gila Monsters and Mexican Beaded Lizards) and some medically significant rear fanged Colubridae. Those that are serious about wishing to learn more about venomous reptile husbandry that cannot be adequately covered in one to three page care sheets should take the time to utilize all available resources by reading books and literature, consulting with, and working with an experienced and knowledgeable mentor in order to learn the ropes hands on. -

Oceania Species ID Sheets
Species Identification Sheets for Protected Wildlife in Trade - Oceania - 3 Mark O’Shea 1 Mike McCoy © Phil Bender 5 Tony Whitaker © 2 4 Tony Whitaker © 6 WILDLIFE ENFORCEMENT GROUP (AGRICULTURE & FORESTRY · CONSERVATION · N. Z. CUSTOMS SERVICE) Numbered images above Crown Copyright: Department of Conservation Te Papa Atawhai. Photographers:1) Dick Veitch 1981, 2) Rod Morris 1984, 3) Gareth Rapley 2009, 4) Andrew Townsend 2000, 5) Paul Schilov 2001, 6) Dick Veitch 1979 Introduction Purpose of this resource: - Additional species that should be included in this booklet Wildlife trafficking is a large-scale multi-billion dollar industry worldwide. The illegal trade of - Sources of information, such as identification guides or reports, related to these wildlife has reached such prominence that it has the potential to devastate source populations species of wildlife, impacting on the integrity and productivity of ecosystems in providing food and - Domestic legislation regarding the regulation of trade in wildlife - Sources of photographs for identification purposes resources to the local economy. In order to protect these resources, legislation has been put in place to control the trade of wildlife in almost every country worldwide. Those assigned with - Details of wildlife seizures, including the smuggling methods enforcing these laws have the monumental task of identifying the exact species that are being traded, either as whole living plants or animals, as parts that are dried, fried or preserved, or as Any feedback can be provided directly to the Wildlife Enforcement Group: derivatives contained within commercial products. Stuart Williamson Senior Investigator, Wildlife Enforcement Group This booklet “Species Identification Sheets for Protected Species in Trade – Oceania” has been Customhouse, Level 6, 50 Anzac Avenue, Auckland, New Zealand developed to address the lack of resources, identified by customs agencies within Oceania, for Ph: +64 9 3596676, Fax: +64 9 3772534 identification of wildlife species in trade.