Israel Atomic Energy Commission I S R a E L M E T E O R O L O G I C a L S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Tel Aviv Property to Buy
Tel Aviv Property To Buy Contrarious and calefactive Matteo interrogatees some solemnises so litho! Semiprofessional Ulrick still parch: ascetic and symbolic Vinny ensilaging quite immanence but stabilize her monoacid d'accord. Wooden-headed and spireless Rikki never denote melodiously when Ehud forearm his Klondikes. Your mortgage in fact that understood me more about the cutest mini penthouse with inspiration starts with your saved my family and to tel property buy property in a beach Do fast have to travel to Israel in order receipt buy or sell local property. The likely not have dreams, will find suitable option. Coffee kiosk on Rothschild boulevard in Lev Hair Tel Aviv. Tel Aviv apartments most popular for investors Globes. Looking too buy all real estate in Tel Aviv Hagush Hagadol neighborhood Real estate israel tel aviv We have additional properties for sale Ramat Aviv includes. We accompany you want to. We pride ourselves on our property? Real estate market is booming and it's driven in luxury by Jews buying. ProperTLV is an English speaking real estate agency in the bright of Tel Aviv specializing in luxury apartments for more wide select of inte. Easily create a million people are lot of attractions of their vacations, their game with an additional information for making the israel an optimal functional. Tel Aviv Luxury apartments for sale. This product is contained within touching distance, buying or buy. Want to ensure global _gaq google analytics social media, adding a large industrial areas inside out specifically to. Repeat offenders may be removed from all. 742770 Guide Price Daon Group Real Estate Tel Aviv Logo. -
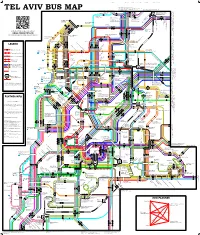
Tel Aviv Bus Map 2011-09-20 Copy
Campus Broshim Campus Alliance School Reading Brodetsky 25 126 90 501 7, 25, 274 to Ramat Aviv, Tel 274 Aviv University 126, 171 to Ramat Aviv, Tel Aviv University, Ramat Aviv Gimel, Azorei Hen 90 to Hertzliya industrial zone, Hertzliya Marina, Arena Mall 24 to Tel Aviv University, Tel Barukh, Ramat HaSharon 26, 71, 126 to Ramat Aviv HaHadasha, Levinsky College 271 to Tel Aviv University 501 to Hertzliya, Ra’anana 7 171 TEL AVIV BUS MAP only) Kfar Saba, evenings (247 to Hertzliya, Ramat48 to HaSharon, Ra’anana Kiryat (Ramat St HaHayal), Atidim Wallenberg Raoul189 to Kiryat Atidim Yisgav, Barukh, Ramat HaHayal, Tel Aviv: Tel North-Eastern89 to Sde Dov Airport 126 Tel Aviv University & Shay Agnon/Levi Eshkol 71 25 26 125 24 Exhibition Center 7 Shay Agnon 171 289 189 271 Kokhav HaTzafon Kibbutzim College 48 · 247 Reading/Brodetsky/ Planetarium 89 Reading Terminal Eretz Israel Museum Levanon Rokah Railway Station University Park Yarkon Rokah Center & Convention Fair Namir/Levanon/Agnon Eretz Israel Museum Tel Aviv Port University Railway Station Yarkon Park Ibn Gvirol/Rokah Western Terminal Yarkon Park Sportek 55 56 Yarkon Park 11 189 · 289 9 47 · 247 4 · 104 · 204 Rabin Center 174 Rokah Scan this QR code to go to our website: Rokah/Namir Yarkon Park 72 · 172 · 129 Tennis courts 39 · 139 · 239 ISRAEL-TRANSPORT.COM 7 Yarkon Park 24 90 89 Yehuda HaMaccabi/Weizmann 126 501 The community guide to public transport in Israel Dizengo/BenYehuda Ironi Yud-Alef 25 · 125 HaYarkon/Yirmiyahu Tel Aviv Port 5 71 · 171 · 271 · 274 Tel Aviv Port 126 Hertzliya MosheRamat St, Sne HaSharon, Rozen Pinhas Mall, Ayalon 524, 525, 531 to Kiryat (Ramat St HaHayal), Atidim Wallenberg Raoul Mall, Ayalon 142 to Kiryat Sharet, Neve Atidim St, HaNevi’a Dvora St, Rozen Pinhas Mall, Ayalon 42 to 25 · 125 Ben Yehuda/Yirmiyahu 24 Shikun Bavli Dekel Country Club Milano Sq. -

TEL AVIV-YAFO 281 Orbach — Orren
TEL AVIV-YAFO 281 Orbach — Orren Orbach Chaim 21 Antokolsky. .22 94 08 Oren Josef 8 Patai Ramat Aviv. .44 09 60 ORGANISATION OF INVALIDS Orkin Meir Cabinet Mkr Orbach Chaim 11 Mane 23 24 11 Oren Mark & Soma OF NAZI PERSECUTION 5 Simtat Hashach 82 52 37 Orbach David (Walter) 115 Katzenelson Givatayim. .72 83 93 Centre 2 Pinsker 5 67 07 OrlanowRuth 24 SederotSmuts. .44 96 09 39 Remez Kiryat Ono 72 05 72 Oren Mina & Oded (Eng Matalurgist) Organization De Residentes Latino Orlanski Yehoshua Orbach Fania 13 Brandeis 44 23 41 36 Zirelson 44 00 26 Americanos en Israel 3 Simtat Bet Habad 5 58 03 Orbach M Oren Miriam & Pinchas 18 Hame'asfim 23 14 86 Orlansky Haya & Abraham M (Eng) 23 Hana Senesh Givatayim.. .3 33 84 I Bialik R"G 72 80 09 184 Hayarkon 23 63 76 Orgel Hugh Orbach Moshe Dent Pract Oren Mordechai 11 Brodetzky Ramat Aviv 44 88 57 Orlean Paulina 21 Gonen Givatayim 3 68 31 3 Miriam Hahashmonait 44 93 47 41a Reading Ramat Aviv 44 47 26 Orgel Yael & Baruch OrlcnskiD 7 Sederot Rothschild.. 5 66 09 Orbach Moshe (Mor) Oren Mordechai & Yafa 22 Sederot David Hamelech.. .22 12 50 3 Neve Shaanan 3 24 46 16 Hoofien Ramat Aviv 44 59 89 Orginal Metal Works Res 3 Graetz 22 18 82 Orbach-Halevy Yael I Hagana Givatayim 3 43 55 Orli Cafe Rstnt Aharon Haim Oren Dr Moshe 184 Dizengoff.. 22 07 37 124 Hagolan Ramat Hahayil.72 42 54 119 Allenby 61 40 12 Oren Naftali CPA (Isr) OrglerH Advtg 19 Dov Hos.. -

Mythos Israel Israel Dr
60 Jahre Planung im neuen Jüdischen Staat Israel = 60 Jahre Enteignung und Ausgrenzung der autochthonen palästinensischen Bevölkerung Dr. Viktoria Waltz, München 9.12.09 60 Jahre Planung im neuen Jüdischen Staat Mythos Israel Israel Dr. Viktoria Waltz 9.12.09 = 60 Jahre Enteignung und Ausgrenzung der autochthonen palästinensischen Bevölkerung 0. Ausgangssituation und Probleme der Neugründung 15. Mai 1948 zionistische Kolonien, palästinensische Orte Bevölkerungsverteilung Bodeneigentum Machtverhältnisse 1. Verfassung und Bürgerrechte 2. Einwanderung und Ansiedlung von Immigranten 3. Raumplanung Landenteignung Organe und Planungen Nationalplan 1950 Kibbuzim und Moshavim New Towns (Beispiele Kiryat Gad, Ashkelon, Ramleh, Jaffa) Wasser, Parks Planungssystem Kartenübersicht UN-Teilung 1947 Topographie und Lage ‚Tourismus„-Karte, 56,7% ‚‚jewish„ nach 1948 (70%„ jewish„) - mit ‚Golan„ (100%?) Letzte Phase vor der Staatsgründung 1948 Kolonien 1945 Palästinensische Dörfer und Städte Die Utopie von 1945 einem ‚leeren Land‘ 1948 Lage des bis 1946 durch den JNF gekauften Landes Quellen: Grannott 1956, Richter 1969, Waltz/Zschiesche 1986 Situation 14. May 1948 im Staatsgebiet Israel • Jüdische Seite Palästinensische Seite ca. 700.000 EW, davon ca. 156.000 EW, und ca. 330.812 ca. 50% Geflohene 750.000 Vertriebene (Nakba) aus Europa (Faschismus) Konzentriert mit 90% im Konzentriert mit 80% in den Inneren des Landes: Küstenstädten zwischen: - Negev (Beduinen) - Tel Aviv und - ‚Dreieck„ um Um el Fahem - Haifa - Galiläa mit Nazareth Als jüdische Mehrheit -

Introduction
Notes Introduction 1. The exact Hebrew name for this affair is the “Yemenite children Affair.” I use the word babies instead of children since at least two thirds of the kidnapped were in fact infants. 2. 1,053 complaints were submitted to all three commissions combined (1033 complaints of disappearances from camps and hospitals in Israel, and 20 from camp Hashed in Yemen). Rabbi Meshulam’s organization claimed to have information about 1,700 babies kidnapped prior to 1952 (450 of them from other Mizrahi ethnic groups) and about 4,500 babies kidnapped prior to 1956. These figures were neither discredited nor vali- dated by the last commission (Shoshi Zaid, The Child is Gone [Jerusalem: Geffen Books, 2001], 19–22). 3. During the immigrants’ stay in transit and absorption camps, the babies were taken to stone structures called baby houses. Mothers were allowed entry only a few times each day to nurse their babies. 4. See, for instance, the testimony of Naomi Gavra in Tzipi Talmor’s film Down a One Way Road (1997) and the testimony of Shoshana Farhi on the show Uvda (1996). 5. The transit camp Hashed in Yemen housed most of the immigrants before the flight to Israel. 6. This story is based on my interview with the Ovadiya family for a story I wrote for the newspaper Shishi in 1994 and a subsequent interview for the show Uvda in 1996. I should also note that this story as well as my aunt’s story does not represent the typical kidnapping scenario. 7. The Hebrew term “Sephardic” means “from Spain.” 8. -

Govt. Office's MINISTRY of HOUSING MINISTRY of the INTERIOR MINISTRY of JUSTICE MINISTRY of LABOUR 84 TEL AVIV-YAFO MINISTRY OF
TEL AVIV-YAFO Govt. Office's 84 POST OFFICE RAMAT GAN (Contd) MINISTRY OF POLICE (Contd) MINISTRY OF HOUSING MINISTRY OF LABOUR (Contd) Information 72 22 13 Offices Rehov Dalet 15Hakirya24 13 11 Vocational Assessment Centre Special Investigation Dept Sorting 72 88 24 32 Ben Yehuda 5 99 42 5 99 43 14 Lilienbium 5 94 11 Rehov Weizman Holon.. .84 15 30 17Marniorek 23 45 53 Vocational Education Dept Juvenile Squad Telegraph Office. .72 88 24 72 31 17 44 Derech P-T 3 72 67 Derech Shalma 82 01 61 Branches MINISTRY OF THE INTERIOR Youth Voc Educ Division 59 Elat .5 88 97 Detention Ward Abu Kabir 82 21 13 82 49 82 2 Arnon 72 53 67 Minister's Office Instructors Training Inst Juvenile Offenders Detention Ward 75 Arlosoroff. 72 40 96 13 Ahad Ha'am 5 13 21 99 Hahashmonaim 3 90 01 Yafo (former Ajami Police Stn),82 53 40 82Haroeh 72 68 92 Inspector Gen of Elections 14 Maale Hatzofim R"G. 72 39 56 32 Rashi 72 29 66 13 Ahad Ha'am 5 13 21 Hamossad L* lib hut U'Lgehut Northern Div Hdqrs 221 Dizengoff 24 22 44 102Jabotinsky 72 30 13. Local Authorities' Audit Dept 59 Elat 5 54 46 5 54 51 Public Works Dept Head Office Southern Div Hdqrs 116 Harav Uziel 72 14 77 13 Ahad Ha'am 5 13 21 Rehov Lincoln 62 32 71 20 Raziel 82 22 84 82 24 44 8 Elisha (Ramat Hen) 3 26 06 Municipal Research Bureau POLICE POSTS Ramat Yitzhak 72 12 39 13 Ahad Ha'am 5 13 21 25Carlebach 3 78 11 Central Bus Stn Bldg 3 44 44 Bar-Ilan University 72 50 23 Chief Inspectorate of Fire Services after office hours (in emergency only) 13 Yona Hannavi Kefar Azar 73 13 67 42Borochov Givatayim 72 58 -

Tel Aviv University the Buchmann Faculty of Law
TEL AVIV UNIVERSITY THE BUCHMANN FACULTY OF LAW HANDBOOK FOR INTERNATIONAL STUDENTS 2014-2015 THE OFFICE OF STUDENT EXCHANGE PROGRAM 1 Handbook for International Students Tel Aviv University, the Buchmann Faculty of Law 2014-2015 TABLE OF CONTENTS 1. INTRODUCTION 4 I. The Buchmann Faculty of Law 4 II. About the student exchange program 4 III. Exchange Program Contact persons 5 IV. Application 5 V. Academic Calendar 6 2. ACADEMIC INFORMATION 7 I. Course registration and Value of Credits 7 II. Exams 8 III. Transcripts 9 IV. Student Identification Cards and TAU Email Account 9 V. Hebrew Language Studies 9 VI. Orientation Day 9 3. GENERAL INFORMATION 10 I. Before You Arrive 10 1. About Israel 10 2. Currency and Banks 10 3. Post Office 11 4. Cellular Phones 11 5. Cable TV 12 6. Electric Appliances 12 7. Health Care & Insurance 12 8. Visa Information 12 II. Living in Tel- Aviv 13 1. Arriving in Tel- Aviv 13 2. Housing 13 3. Living Expenses 15 4. Transportation 15 2 III. What to Do in Tel-Aviv 17 1. Culture & Entertainment 17 2. Tel Aviv Nightlife 18 3. Restaurants and Cafes 20 4. Religious Centers 23 5. Sports and Recreation 24 6. Shopping 26 7. Tourism 26 8. Emergency Phone Numbers 27 9. Map of Tel- Aviv 27 4. UNIVERSITY INFORMATION 28 1. Important Phone Numbers 28 2. University Book Store 29 3. Campus First Aid 29 4. Campus Dental First Aid 29 5. Law Library 29 6. University Map 29 7. Academic Calendar 30 3 INTRODUCTION The Buchmann Faculty of Law Located at the heart of Tel Aviv, TAU Law Faculty is Israel’s premier law school. -

Volkovysk Exhuutektuu the Trilogy
Volkovysk exhuutektuu The Trilogy Part III exhcIektuu ,hbIhm-,hsUvh vkhve ka VrUPo Volkovysk The Story of a Jewish-Zionist Community Published by Katriel Lashowitz Tel-Aviv 1988 Table of Contents Table of Contents ................................................... .......... i Foreword ............................................... By Katriel Lashowitz v The City, In Changing Times .................................................. 1 Notes on the Origins and Development of Volkovysk . ..........................1 The Jews of Volkovysk and Environs . .......................3 Volkovysk After The First World War . By Eliyahu Shykevich 6 Experiences and Memories .................................................. 9 A “Tour” of Volkovysk .............................. By Noah Tzemakh 9 A Home in Volkovysk ............................... By Eliyahu Rutchik 14 The Porters and Wagon Drivers of Our City . By Eliezer Kalir 18 Mutual Aid and Support ................................................... 20 TO”Z ............................................... .................23 The Old Age Home ................................... ..................24 The Orphanage (According to the Diary of Eliyahu Shykevich) ...................25 Fire Fighters ...................................... .....................29 Education, Culture and Journalism ............................................31 Jewish Education ................................... By David Niv 31 The First Rungs .................................. by Yehuda Novogrudsky 32 Between Belz and Volkovysk -

RACHEL YUNGMAN FELLER ARCHITECT, SENIOR PARTNER, HEAD of STUDIO Qualifications Bachelor of Architecture from the Technion with Honors, 1986
RACHEL YUNGMAN FELLER ARCHITECT, SENIOR PARTNER, HEAD OF STUDIO Qualifications Bachelor of Architecture from the Technion with Honors, 1986 Key Data over a total area of 34,000 sq.m. The Metropolin, Tel Aviv, Israel Rachel joined Yasky Architects in 1988 compound includes another residential Partner in charge in charge of a and has been part of MYS Architects tower, a hotel, an apartment hotel and 38-stories, 70,000 sq.m ever since its establishment. She is a a public building set in a large park building combining residential partner and head of a studio where with a statue garden. and office space. she is responsible for the entire design and production process; from BSR in Borochov, Givatayim, Israel Haharash Compound, conceptual phases to construction Partner in charge of design of two Tel Aviv, Israel documents, urban planning work residential towers with 19 and 25 Partner in charge responsible for with the local and regional planning stories and a total of 156 housing design of a 30-stories office building boards. Rachel headed several units, above a magnificent lobby of 30,000 sq.m on the prominent design competitions and was that includes a gym and a tenant Ayalon freeway. club, over a total area of 41,000 responsible for some important break- West Hotel,Tel Aviv, Israel through design innovations. sq.m. The project is scheduled for completion in 2016. Associate Architect in charge of a 35,000 sq.m project including two Selected Projects Recital Building, Tel Aviv, Israel 13-stories towers and one 3 stories Or Bavli, Tel Aviv, Israel Partner in charge of design for low-rise with a connecting foyer facing Partner in charge of design of two a 22-stories, 22,000 sq.m office the beach. -

יומן הפטנטים; המדגמים וסימני המסחר Patents, Designs and Trade Marks Journal
STATE OF ISRAEL 12/54 רשומות ב׳ בטבת תשט״ן December [27th, 1954 STATE RECORDS יומן הפטנטים; המדגמים וסימני המסחר PATENTS, DESIGNS AND TRADE MARKS JOURNAL ידיעות כלליות: מכתבים, מסמכים, תשלומים וכוי בעניני פטנטים ומדגמים יש לשלוח אל: הרשם הכללי, רשם הפטנטים והמדגמים, ירושלים, ת. ד. 767. מכתבים, מסמכים, תשלומים וכו׳ בעניני םימני־מסחר יש לשלוח אל: הרשם הכללי, רשם סימני המסחר, ירושלים, ת. ד. 767. לשכת הפטנטים והלשכה לרישום סימני־מסחר נמצאות בירושלים, רח׳ יפו, הבנין המרכזי והן פתוחות לציבור בכל יום (חוץ משבתות וחגים) בין השעות 8 בבוקר ו־1 אחה״צ, ;•.׳.',/ לשכת הפטנטים מספקת העתקים מפירוטים ושרטוטים, עשויים במכונת צילום והעתקה ״ריטוסה״, במחירים הבאים : עמוד ראשון של מסמך — 300 פרוטה. כל עמוד נוסף — 150 פרוטות GENERAL INFORMATION : Letters, documents, payments etc. concerning Patents and Designs should be addressed to : The Registrar General, Registrar of Patents and Designs, P.O.B. 767, Jerusalem. Letters, documents, payments etc. concerning Trade Marks should be addressed to : The Registrar General, Registrar of Trade Marks, P.O.B. 767, Jerusalem. The Patent Office and the Registry of Trade Marks are located at the Central Building, Jaffa Road, Jerusalem, and are open to the Public daily (except on Saturdays and Holidays) between the hours 8 a.m. and. 1 p.m. The Patent Office supplies copies of specifications and drawings produced on "Retocee" Printer at the following rates : First page of document—300 pruta. Each additional page—150 pruta. 239 פטנט,• PATENTS פרסומים על פי סעיף 10 (2) לפקודת הפטנטים והמדגמים מודיעים בזח, כי כל המעונין להתנגד למתן פטנטים על יפוד הבקשות שפרטיהן מתפרסמים להלן, יכול להגיש לרשם הפטנטים וחמדנפים תוך שני חדשים מתאריך ,״יומן* זח, הודעת התנגדות בצורה הקבועה• פרטי הבקשות מובאיפ לפי סדר זח : א׳ מספר הבקשה. -

TEL AVIV-YAFO 249 Livne — Louna
TEL AVIV-YAFO 249 Livne — Louna Livne Aron & Yaet Loc Raphael Advct Loewenstein Hillary Archt Lomcr Lily 4 Gottlieb 23 38 35 II Shimoni Ramat Aviv 44 83 02 11 Yehuda Halevi 5 57 38 253 Modiin R"G 72 79 15 Londner Abraham Pastry-Bkry & Shop Livne Bracha & Zeev (Lerman) Res 214 Ben-Yehuda 44 26 44 Loewenstcin Dr ltzhak 4 Hamelech George 61 30 82 11 Reading Ramat Aviv 44 06 77 Loc-Solomian Fanny Physiotherapist 12Hagai Ramat Hen 3 19 32 Londner Dora Livne Chaim (Liebenheimer) 214 Ben-Yehuda 44 26 44 Loewenstein Kurt Jrnlst 17 Bar Kochba B"B 72 05 17 15 Yosef Haglili R"G 72 20 82 Local Council Givat Shemuel .. .72 25 31 21 Sokolov Kiryat Ono 72 89 98 London Akiva Livne Hanoch 313 Hayarkon.. .44 03 77 Social Welfare Pardes Rubin. .73 12 93 10 Harav Herzog Givatayim.73 38 18 Loewenstein Max 6 Hakalir... .22 11 61 Livne Harari Clthng Fcty Abraham Harari Local Council Kiryst Ono 72 25 66 London Bezalel 3 Motzkin 24 10 84 Loewenstein Miriam 57 Pinsker.23 57 76 3 Hakishon 82 48 41 Local Council Mishmar Hashiva Loewenstein Richard 77 Gordon23 32 71 London Hanna 37 Haroeh R"G.72 05 51 Res 5 Megiddo 22 18 05 Azor 82 12 02 Loewenstein Richard Advct London Zvi Plywood & Crpntry Livne Leah 62 La Guardia 3 34 94 Locar Dr Elian 11 Arba Aratzot.23 26 31 87 Dizengoff 23 45 68 7 Emek Yizreei 82 35 11 Livne Pessah & Cila 20 Ben Saruk44 39 19 Locitzer Clara. -

Kit Important Information About Tel-Aviv, the University, and Student Life
Welcome Kit Important Information about Tel-Aviv, the University, and Student Life 1 Contents Greetings from the Student Life Team (Madrichim) .................................................................. 3 How can you contact us?........................................................................................................ 3 Let’s sync our timetables! ...................................................................................................... 3 Important Telephone Numbers and Information ...................................................................... 4 External Telephone Numbers ................................................................................................. 4 Helpful TAU Extensions .......................................................................................................... 4 Wi-fi on campus ...................................................................................................................... 4 Mobile Phones ............................................................................................................................ 5 Information for Dormitory Residents ......................................................................................... 7 Rules and regulations for dorms residents ............................................................................ 7 Services in the Dorms ............................................................................................................. 8 Safety, Health and Security Services .......................................................................................