16 Fruiting-Body Development in Ascomycetes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution of Genetic Systems in Filamentous Ascomycetes
Evolution of Genetic Systems in Filamentous Ascomycetes Evolutie van genetische systemen in hyphenvormende zakjeszwammen 0000 0513 3836 Promotor: dr. R.F. Hoekstra hoogleraar in de populatie- en kwantitatieve genetica fjtfoiißi f ßin Maarten J. Nauta Evolution of Genetic Systems in Filamentous Ascomycetes Proefschrift ter verkrijging van de graad van doctor in de landbouw- en milieuwetenschappen op gezag van de rector magnificus, dr. C.M. Karssen, in het openbaar te verdedigen op woensdag 12januar i 1994 des namiddags te vier uur in de Aula van de Landbouwuniversiteit te Wageningen. 15 0 S(p^ZJ> These investigations were supported by the Netherlands Organization for Scientific Research (N.W.O.). BibUt/FHEEK LAMDbOirWUNIVERSITEJi. WAGE NINGE N CIP-GEGEVENS KONINKLIJKE BIBLIOTHEEK, DEN HAAG Nauta, Maarten J. Evolution of genetic systems in filamentous ascomycetes / Maarten J. Nauta. - [ S.l. : s.n.]. -111 . Thesis Wageningen. - With ref. - With summary in Dutch. ISBN 90-5485-199-6 Subject headings: population genetics / ascomycetes. omslagontwerp: Ernst van Cleef foto omslag: Barrages tussen verschillende stammen van Podospora anserina als gevolg van vegetatieve incompatibiliteit. (met dank aan Inge Haspels) aan mijn ouders Voorwoord Dit proefschrift is het resultaat van vier jaar onderzoek, verricht bij de vakgroep Erfelijkheidsleer van de Landbouwuniversiteit in Wageningen. In zekere zin valt zo'n proefschrift te vergelijken met een levend wezen. Uit de genetica is bekend dat de verschijningsvorm van elk levend wezen tot stand komt door een combinatie van erfelijke aanleg en invloeden uit de omgeving. Voor een proefschrift geldt eigenlijk hetzelfde: Zowel het werk van de auteur, als de bijdragen van zijn omgeving zijn onontbeerlijk om tot een verschijningsvorm te komen. -

Phylogenetic Investigations of Sordariaceae Based on Multiple Gene Sequences and Morphology
mycological research 110 (2006) 137– 150 available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/mycres Phylogenetic investigations of Sordariaceae based on multiple gene sequences and morphology Lei CAI*, Rajesh JEEWON, Kevin D. HYDE Centre for Research in Fungal Diversity, Department of Ecology & Biodiversity, The University of Hong Kong, Pokfulam Road, Hong Kong SAR, PR China article info abstract Article history: The family Sordariaceae incorporates a number of fungi that are excellent model organisms Received 10 May 2005 for various biological, biochemical, ecological, genetic and evolutionary studies. To deter- Received in revised form mine the evolutionary relationships within this group and their respective phylogenetic 19 August 2005 placements, multiple-gene sequences (partial nuclear 28S ribosomal DNA, nuclear ITS ribo- Accepted 29 September 2005 somal DNA and partial nuclear b-tubulin) were analysed using maximum parsimony and Corresponding Editor: H. Thorsten Bayesian analyses. Analyses of different gene datasets were performed individually and Lumbsch then combined to generate phylogenies. We report that Sordariaceae, with the exclusion Apodus and Diplogelasinospora, is a monophyletic group. Apodus and Diplogelasinospora are Keywords: related to Lasiosphaeriaceae. Multiple gene analyses suggest that the spore sheath is not Ascomycota a phylogenetically significant character to segregate Asordaria from Sordaria. Smooth- Gelasinospora spored Sordaria species (including so-called Asordaria species) constitute a natural group. Neurospora Asordaria is therefore congeneric with Sordaria. Anixiella species nested among Gelasinospora Sordaria species, providing further evidence that non-ostiolate ascomata have evolved from ostio- late ascomata on several independent occasions. This study agrees with previous studies that show heterothallic Neurospora species to be monophyletic, but that homothallic ones may have a multiple origins. -

Self-Fertility and Uni-Directional Mating-Type Switching in Ceratocystis Coerulescens, a Filamentous Ascomycete
Curr Genet (1997) 32: 52–59 © Springer-Verlag 1997 ORIGINAL PAPER T. C. Harrington · D. L. McNew Self-fertility and uni-directional mating-type switching in Ceratocystis coerulescens, a filamentous ascomycete Received: 6 July 1996 / 25 March 1997 Abstract Individual perithecia from selfings of most some filamentous ascomycetes. Although a switch in the Ceratocystis species produce both self-fertile and self- expression of mating-type is seen in these fungi, it is not sterile progeny, apparently due to uni-directional mating- clear if a physical movement of mating-type genes is in- type switching. In C. coerulescens, male-only mutants of volved. It is also not clear if the expressed mating-types otherwise hermaphroditic and self-fertile strains were self- of the respective self-fertile and self-sterile progeny are sterile and were used in crossings to demonstrate that this homologs of the mating-type genes in other strictly heter- species has two mating-types. Only MAT-2 strains are othallic species of ascomycetes. capable of selfing, and half of the progeny from a MAT-2 Sclerotinia trifoliorum and Chromocrea spinulosa show selfing are MAT-1. Male-only, MAT-2 mutants are self- a 1:1 segregation of self-fertile and self-sterile progeny in sterile and cross only with MAT-1 strains. Similarly, self- perithecia from selfings or crosses (Mathieson 1952; Uhm fertile strains generally cross with only MAT-1 strains. and Fujii 1983a, b). In tetrad analyses of selfings or crosses, MAT-1 strains only cross with MAT-2 strains and never self. half of the ascospores in an ascus are large and give rise to It is hypothesized that the switch in mating-type during self-fertile colonies, and the other ascospores are small and selfing is associated with a deletion of the MAT-2 gene. -

Fungal Cannons: Explosive Spore Discharge in the Ascomycota Frances Trail
MINIREVIEW Fungal cannons: explosive spore discharge in the Ascomycota Frances Trail Department of Plant Biology and Department of Plant Pathology, Michigan State University, East Lansing, MI, USA Correspondence: Frances Trail, Department Abstract Downloaded from https://academic.oup.com/femsle/article/276/1/12/593867 by guest on 24 September 2021 of Plant Biology, Michigan State University, East Lansing, MI 48824, USA. Tel.: 11 517 The ascomycetous fungi produce prodigious amounts of spores through both 432 2939; fax: 11 517 353 1926; asexual and sexual reproduction. Their sexual spores (ascospores) develop within e-mail: [email protected] tubular sacs called asci that act as small water cannons and expel the spores into the air. Dispersal of spores by forcible discharge is important for dissemination of Received 15 June 2007; revised 28 July 2007; many fungal plant diseases and for the dispersal of many saprophytic fungi. The accepted 30 July 2007. mechanism has long been thought to be driven by turgor pressure within the First published online 3 September 2007. extending ascus; however, relatively little genetic and physiological work has been carried out on the mechanism. Recent studies have measured the pressures within DOI:10.1111/j.1574-6968.2007.00900.x the ascus and quantified the components of the ascus epiplasmic fluid that contribute to the osmotic potential. Few species have been examined in detail, Editor: Richard Staples but the results indicate diversity in ascus function that reflects ascus size, fruiting Keywords body type, and the niche of the particular species. ascus; ascospore; turgor pressure; perithecium; apothecium. 2 and 3). Each subphylum contains members that forcibly Introduction discharge their spores. -

MEIOSIS and RECOMBINATION in SORDARIA FIMICOLA Introduction
MEIOSIS AND RECOMBINATION IN SORDARIA FIMICOLA Introduction: In ascomycete fungi, a form of meiosis occurs in which the products of meiosis order themselves within a fruiting body according to the physical separation and segregation of chromatids during the meiotic process. This is covered in some detail on pages 150-152 (including Figures 4.26 and 4.27) in Hartl and Jones, Essential Genetics. You should study these pages before beginning this module. As described, ordered tetrad analysis provides a way to measure the genetic map distance between a gene and the centromere of the chromosome on which that gene resides. That is what you will do over the next two weeks in this laboratory. I. Natural history and Life Cycle of Sordaria fimicola Sordaria fimicola is an ascomycete fungi that can be found growing in rotting vegetation and animal dung (in fact, the name Sordaria fimicola means "filthy dung dweller"). Sordaria and another ascomycete, the common bread fungus Neurospora crassa (Fig. 4.26), have been used as model systems for studying the process of chromosome exchange (crossing-over) because of their reproductive characteristics. The life cycle of Sordaria is representative of the ascomycetes (although there are substantial differences in the details among species). The individual fungus begins as a haploid ascospore. The ascospore germinates to form hyphae (singular = hypha), which are long filaments comprised of haploid cells. These hyphae grow and extend throughout the nutrient source (dung or rotting vegetation in nature, nutrient medium in the laboratory situation) and digest it by means of enzymes secreted by the cells. Nutrients are then absorbed into the cells. -

Advanced Gene Mapping in Eukaryotes
RUSSMC07 2/7/05 11:54 AM Page 165 7 Advanced Gene Mapping in Eukaryotes Ordered tetrads of the fungus, Neurospora Crassa. PRINCIPAL POINTS • Tetrad analysis is a mapping technique that can be be used to determine gene order and map distances used to map the genes of certain haploid eukaryotic between genes. organisms in which the products of a single meiosis— • Due to both ethical and practical issues, human the meiotic tetrad—are contained within a single genes cannot be mapped by making crosses and structure. In these situations, the map distance analyzing progeny. A number of approaches are between genes is computed by analyzing the relative used to determine the linkage relationships proportion of tetrad types, rather than by analyzing between human genes, including analyzing individual progeny. pedigree data recombinationally and physically • In organisms in which the meiotic tetrads are locating genes on chromosomes by molecularly arranged linearly, it is easy to map the distance aided methods. of a gene from its centromere. • Crossing-over can also occur during mitosis, although i SPECIAL TECHNIQUES ARE AVAILABLE FOR at a frequency much lower than during meiosis. As in constructing linkage maps for eukaryotic organisms meiosis, mitotic crossing-over occurs at a four-strand beyond the standard genetic mapping crosses. In this stage. chapter you will learn about these techniques. Then, in the iActivity, you can apply what you’ve learned and further • For organisms amenable to such analysis, such as the explore one of the advanced mapping techniques. fungus Aspergillus nidulans, mitotic recombination can iActivity 165 RUSSMC07 1/26/05 12:47 PM Page 166 166 Chapter 7 Advanced Gene Mapping in Eukaryotes In the previous chapter, we considered the classical princi- Go to the iActivity Mapping Genes by Tetrad i ples for mapping genes in eukaryotes by means of recombi- Analysis on your CD-ROM and assume the role nation analysis. -

Coprophilous Fungal Community of Wild Rabbit in a Park of a Hospital (Chile): a Taxonomic Approach
Boletín Micológico Vol. 21 : 1 - 17 2006 COPROPHILOUS FUNGAL COMMUNITY OF WILD RABBIT IN A PARK OF A HOSPITAL (CHILE): A TAXONOMIC APPROACH (Comunidades fúngicas coprófilas de conejos silvestres en un parque de un Hospital (Chile): un enfoque taxonómico) Eduardo Piontelli, L, Rodrigo Cruz, C & M. Alicia Toro .S.M. Universidad de Valparaíso, Escuela de Medicina Cátedra de micología, Casilla 92 V Valparaíso, Chile. e-mail <eduardo.piontelli@ uv.cl > Key words: Coprophilous microfungi,wild rabbit, hospital zone, Chile. Palabras clave: Microhongos coprófilos, conejos silvestres, zona de hospital, Chile ABSTRACT RESUMEN During year 2005-through 2006 a study on copro- Durante los años 2005-2006 se efectuó un estudio philous fungal communities present in wild rabbit dung de las comunidades fúngicas coprófilos en excementos de was carried out in the park of a regional hospital (V conejos silvestres en un parque de un hospital regional Region, Chile), 21 samples in seven months under two (V Región, Chile), colectándose 21 muestras en 7 meses seasonable periods (cold and warm) being collected. en 2 períodos estacionales (fríos y cálidos). Un total de Sixty species and 44 genera as a total were recorded in 60 especies y 44 géneros fueron detectados en el período the sampling period, 46 species in warm periods and 39 de muestreo, 46 especies en los períodos cálidos y 39 en in the cold ones. Major groups were arranged as follows: los fríos. La distribución de los grandes grupos fue: Zygomycota (11,6 %), Ascomycota (50 %), associated Zygomycota(11,6 %), Ascomycota (50 %), géneros mitos- mitosporic genera (36,8 %) and Basidiomycota (1,6 %). -

Six Key Traits of Fungi: Their Evolutionary Origins and Genetic Bases LÁSZLÓ G
Six Key Traits of Fungi: Their Evolutionary Origins and Genetic Bases LÁSZLÓ G. NAGY,1 RENÁTA TÓTH,2 ENIKŐ KISS,1 JASON SLOT,3 ATTILA GÁCSER,2 and GÁBOR M. KOVÁCS4,5 1Synthetic and Systems Biology Unit, Institute of Biochemistry, HAS, Szeged, Hungary; 2Department of Microbiology, University of Szeged, Szeged, Hungary; 3Department of Plant Pathology, Ohio State University, Columbus, OH 43210; 4Department of Plant Anatomy, Institute of Biology, Eötvös Loránd University, Budapest, Hungary; 5Plant Protection Institute, Center for Agricultural Research, Hungarian Academy of Sciences, Budapest, Hungary ABSTRACT The fungal lineage is one of the three large provides an overview of some of the most important eukaryotic lineages that dominate terrestrial ecosystems. fungal traits, how they evolve, and what major genes They share a common ancestor with animals in the eukaryotic and gene families contribute to their development. The supergroup Opisthokonta and have a deeper common ancestry traits highlighted here represent just a sample of the with plants, yet several phenotypes, such as morphological, physiological, or nutritional traits, make them unique among characteristics that have evolved in fungi, including po- all living organisms. This article provides an overview of some of larized multicellular growth, fruiting body development, the most important fungal traits, how they evolve, and what dimorphism, secondary metabolism, wood decay, and major genes and gene families contribute to their development. mycorrhizae. However, a great deal of other important The traits highlighted here represent just a sample of the traits also underlie the evolution of the taxonomically characteristics that have evolved in fungi, including polarized and phenotypically hyperdiverse fungal kingdom, which multicellular growth, fruiting body development, dimorphism, could fill up a volume on its own. -

Integrative Activity of Mating Loci, Environmentally Responsive Genes, and Secondary Metabolism Pathways During Chaetomium Globosum Sexual Development
Integrative activity of mating loci, environmentally responsive genes, and secondary metabolism pathways during Chaetomium globosum sexual development Zheng Wang1, Francesc López-Giráldez2, Junrui Wang1, Frances Trail5, Jeffrey P. Townsend1,4,5 1 Department of Biostatistics, Yale University, New Haven, CT 06510, USA 2 Yale Center for Genome Analysis (YCGA) and Department of Genetics, Yale University, New Haven, CT 06511, USA 3 Department of Plant, Soil and Microbial Sciences, Michigan State University, East Lansing, MI 48824, USA 4 Department of Ecology and Evolutionary Biology, Yale University, New Haven, CT 06520, USA 5 Program in Computational Biology and Bioinformatics, Yale University, New Haven, CT 06511, USA Running title: Chaetomium sexual development Abstract The origins and maintenance of the rich morphological and ecological diversity of fungi has been a longstanding question in evolutionary biology. To investigate how differences in expression regulation contribute to divergences in development and ecology among closely related species, comparative transcriptomics was applied to Chaetomium globosum and previously studied model species of Neurospora and Fusarium, which represent diversity from saprotrophic to pathogenetic biology, from post-fire terrestrial to highly humid ecology, and from heterothallic, pseudo-homothallic to homothallic lifestyles. Gene expression was quantified in perithecia at nine distinct morphological stages during nearly synchronous sexual development. Unlike N. crassa, expression of all mating loci in C. globosum was highly correlated. Key regulators of the initiation of sexual development in response to light stimuli—including orthologs of N. crassa sub-1, sub-1-dependent gene NCU00309, and asl-1—showed regulatory dynamics matching between C. globosum and N. crassa. Among 24 secondary-metabolism gene clusters in C. -

Molecular Systematics of the Sordariales: the Order and the Family Lasiosphaeriaceae Redefined
Mycologia, 96(2), 2004, pp. 368±387. q 2004 by The Mycological Society of America, Lawrence, KS 66044-8897 Molecular systematics of the Sordariales: the order and the family Lasiosphaeriaceae rede®ned Sabine M. Huhndorf1 other families outside the Sordariales and 22 addi- Botany Department, The Field Museum, 1400 S. Lake tional genera with differing morphologies subse- Shore Drive, Chicago, Illinois 60605-2496 quently are transferred out of the order. Two new Andrew N. Miller orders, Coniochaetales and Chaetosphaeriales, are recognized for the families Coniochaetaceae and Botany Department, The Field Museum, 1400 S. Lake Shore Drive, Chicago, Illinois 60605-2496 Chaetosphaeriaceae respectively. The Boliniaceae is University of Illinois at Chicago, Department of accepted in the Boliniales, and the Nitschkiaceae is Biological Sciences, Chicago, Illinois 60607-7060 accepted in the Coronophorales. Annulatascaceae and Cephalothecaceae are placed in Sordariomyce- Fernando A. FernaÂndez tidae inc. sed., and Batistiaceae is placed in the Euas- Botany Department, The Field Museum, 1400 S. Lake Shore Drive, Chicago, Illinois 60605-2496 comycetes inc. sed. Key words: Annulatascaceae, Batistiaceae, Bolini- aceae, Catabotrydaceae, Cephalothecaceae, Ceratos- Abstract: The Sordariales is a taxonomically diverse tomataceae, Chaetomiaceae, Coniochaetaceae, Hel- group that has contained from seven to 14 families minthosphaeriaceae, LSU nrDNA, Nitschkiaceae, in recent years. The largest family is the Lasiosphaer- Sordariaceae iaceae, which has contained between 33 and 53 gen- era, depending on the chosen classi®cation. To de- termine the af®nities and taxonomic placement of INTRODUCTION the Lasiosphaeriaceae and other families in the Sor- The Sordariales is one of the most taxonomically di- dariales, taxa representing every family in the Sor- verse groups within the Class Sordariomycetes (Phy- dariales and most of the genera in the Lasiosphaeri- lum Ascomycota, Subphylum Pezizomycotina, ®de aceae were targeted for phylogenetic analysis using Eriksson et al 2001). -

Diversity and Distribution Patterns of Endolichenic Fungi in Jeju Island, South Korea
sustainability Article Diversity and Distribution Patterns of Endolichenic Fungi in Jeju Island, South Korea Seung-Yoon Oh 1,2 , Ji Ho Yang 1, Jung-Jae Woo 1,3, Soon-Ok Oh 3 and Jae-Seoun Hur 1,* 1 Korean Lichen Research Institute, Sunchon National University, 255 Jungang-Ro, Suncheon 57922, Korea; [email protected] (S.-Y.O.); [email protected] (J.H.Y.); [email protected] (J.-J.W.) 2 Department of Biology and Chemistry, Changwon National University, 20 Changwondaehak-ro, Changwon 51140, Korea 3 Division of Forest Biodiversity, Korea National Arboretum, 415 Gwangneungsumok-ro, Pocheon 11186, Korea; [email protected] * Correspondence: [email protected]; Tel.: +82-61-750-3383 Received: 24 March 2020; Accepted: 1 May 2020; Published: 6 May 2020 Abstract: Lichens are symbiotic organisms containing diverse microorganisms. Endolichenic fungi (ELF) are one of the inhabitants living in lichen thalli, and have potential ecological and industrial applications due to their various secondary metabolites. As the function of endophytic fungi on the plant ecology and ecosystem sustainability, ELF may have an influence on the lichen diversity and the ecosystem, functioning similarly to the influence of endophytic fungi on plant ecology and ecosystem sustainability, which suggests the importance of understanding the diversity and community pattern of ELF. In this study, we investigated the diversity and the factors influencing the community structure of ELF in Jeju Island, South Korea by analyzing 619 fungal isolates from 79 lichen samples in Jeju Island. A total of 112 ELF species was identified and the most common species belonged to Xylariales in Sordariomycetes. -
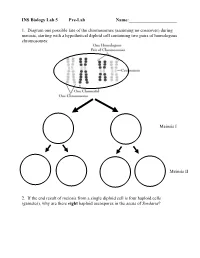
INS Biology Lab 5 Pre-Lab Name:______
INS Biology Lab 5 Pre-Lab Name:_____________________ 1. Diagram one possible fate of the chromosomes (assuming no crossover) during meiosis, starting with a hypothetical diploid cell containing two pairs of homologous chromosomes: Meiosis I Meiosis II 2. If the end result of meiosis from a single diploid cell is four haploid cells (gametes), why are there eight haploid ascospores in the ascus of Sordaria? 2 Winter Lab 1. Meiosis This lab exercise courtesy of Mike Clayton, University of Wisconsin Adapted from a lab written by Jon Glase, Cornell University I. The Process of Meiosis Meiosis is a process of two sequenced nuclear divisions neither of which is identical to mitosis. Meiosis always begins with a nucleus with two sets of chromosomes (a diploid nucleus) where each chromosome has two chromatids. Meiosis produces four nuclei each with one set of chromosomes (haploid nuclei) in which each chromosome has one chromatid. Meiosis together with syngamy constitutes sexuality in eukaryotes. Sexual reproduction generates individuals with new and unique genotypic combinations each generation. Meiosis, by itself, can generate new combinations of genetic material. It does so in two ways. -- through the independent assortment of different homologous pairs of chromosomes and -- through crossing over between homologous pairs of chromosomes. Pop Beads as Models of Chromosomes The shuffling and recombination of genetic material can be modeled using pop beads. Before lab, preview this web page to see a demonstration of meiosis using pop beads ( http://botit.botany.wisc.edu/courses/botany_130/Meiosis/pop.html ). In lab, you will work with a partner to recreate the following scenarios of meiosis..