Envhydrogeology Francistown
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LIST of LAW FIRMS NAME of FIRM ADDRESS TEL # FAX # Ajayi Legal
THE LAW SOCIETY OF BOTSWANA - LIST OF LAW FIRMS NAME OF FIRM ADDRESS TEL # FAX # Ajayi Legal Chambers P.O.Box 228, Sebele 3111321 Ajayi Legal Chambers P.O.Box 10220, Selebi Phikwe 2622441 2622442 Ajayi Legal Chambers P.O.Box 449, Letlhakane 2976784 2976785 Akheel Jinabhai & Associates P.O.Box 20575, Gaborone 3906636/3903906 3906642 Akoonyatse Law Firm P.O. Box 25058, Gaborone 3937360 3937350 Antonio & Partners Legal Practice P.O.Box HA 16 HAK, Maun 6864475 6864475 Armstrong Attorneys P.O.Box 1368, Gaborone 3953481 3952757 Badasu & Associates P.O.Box 80274, Gaborone 3700297 3700297 Banyatsi Mmekwa Attorneys P.O.Box 2278 ADD Poso House, Gaborone 3906450/2 3906449 Baoleki Attorneys P.O.Box 45111 Riverwalk, Gaborone 3924775 3924779 Bayford & Botha Attorneys P.O.Box 390, Lobatse 5301369 5301370 B. Maripe & Company P.O.Box 1425, Gaborone 3903258 3181719 B.K.Mmolawa Attorneys P.O.Box 30750, Francistown 2415944 2415943 Bayford & Associates P.O.Box 202283, Gaborone 3956877 3956886 Begane & Associates P.O. Box 60230, Gaborone 3191078 Benito Acolatse Attorneys P.O.Box 1157, Gaborone 3956454 3956447 Bernard Bolele Attorneys P.O.Box 47048, Gaborone 3959111 3951636 Biki & Associates P.O.Box AD 137ABE, Gaborone 3952559 Bogopa Manewe & Tobedza Attorneys P.O.Box 26465, Gaborone 3905466 3905451 Bonner Attorneys Bookbinder Business Law P/Bag 382, Gaborone 3912397 3912395 Briscoe Attorneys P.O.Box 402492, Gaborone 3953377 3904809 Britz Attorneys 3957524 3957062 Callender Attorneys P.O.Box 1354, Francistown 2441418 2446886 Charles Tlagae Attorneys P.O.Box -

2011 Population and Housing Census
2011 POPULATION AND HOUSING CENSUS Ministry of Finance and Development Planning 2011 Census Slogan: Palo yame, tsela ya ditlhabololo My count, a guide to developments August 2009 CSO in Collaboration with UNFPA PROJECT DOCUMENT 2011 POPULATION AND HOUSING CENSUS Published by Central Statistics Office Private Bag 0024, Gaborone Telephone: 33671300 Fax: 3952201 E-mail:[email protected] Website: www.cso.gov.bw Contact Unit : Census Secretariat Telephone: 3671300 Ext. 1305 August 2009 COPYRIGHT RESERVED Extracts may be published if source is duly acknowledged i CONTENTS PREFACE .................................................................................................................... 1 LIST OF ABBREVIATIONS AND ACRONYMS ....................................................... 2 EXECUTIVE SUMMARY ........................................................................................... 4 1. BACKGROUND AND JUSTIFICATION ................................................................ 5 1.1 Background of the 2011 Census Taking .............................................................. 5 1.2 Justification ......................................................................................................... 5 1.2.1 Evidence-based decision making, policy-making, planning and administration ....................................................................................................... 5 1.2.2 Research .................................................................................................... 5 1.2.3 Service to stakeholders -

Botswana Environment Statistics Water Digest 2018
Botswana Environment Statistics Water Digest 2018 Private Bag 0024 Gaborone TOLL FREE NUMBER: 0800600200 Tel: ( +267) 367 1300 Fax: ( +267) 395 2201 E-mail: [email protected] Website: http://www.statsbots.org.bw Published by STATISTICS BOTSWANA Private Bag 0024, Gaborone Phone: 3671300 Fax: 3952201 Email: [email protected] Website: www.statsbots.org.bw Contact Unit: Environment Statistics Unit Phone: 367 1300 ISBN: 978-99968-482-3-0 (e-book) Copyright © Statistics Botswana 2020 No part of this information shall be reproduced, stored in a Retrieval system, or even transmitted in any form or by any means, whether electronically, mechanically, photocopying or otherwise, without the prior permission of Statistics Botswana. BOTSWANA ENVIRONMENT STATISTICS WATER DIGEST 2018 Statistics Botswana PREFACE This is Statistics Botswana’s annual Botswana Environment Statistics: Water Digest. It is the first solely water statistics annual digest. This Digest will provide data for use by decision-makers in water management and development and provide tools for the monitoring of trends in water statistics. The indicators in this report cover data on dam levels, water production, billed water consumption, non-revenue water, and water supplied to mines. It is envisaged that coverage of indicators will be expanded as more data becomes available. International standards and guidelines were followed in the compilation of this report. The United Nations Framework for the Development of Environment Statistics (UNFDES) and the United Nations International Recommendations for Water Statistics were particularly useful guidelines. The data collected herein will feed into the UN System of Environmental Economic Accounting (SEEA) for water and hence facilitate an informed management of water resources. -

Botswana COVID-19 Guideline 5: Quarantine and Isolation
Botswana COVID-19 Guideline 5: Quarantine and Isolation Version: 1.0 5th May 2020 Table of Contents FOREWORD ............................................................................. Error! Bookmark not defined. ACKNOWLEDGEMENTS ................................................................................................... 3 DEFINITION OF TERMS .................................................................................................. 5 ABBREVIATIONS AND ACRONYMS .............................................................................. 6 SCOPE OF THE DOCUMENT ............................................................................................ 7 CASE DEFINITIONS ......................................................................................................... 8 PRINCIPLES OF QUARANTINE...................................................................................... 9 INSTITUTIONAL QUARANTINE .................................................................................. 11 HOME QUARANTINE ...................................................................................................... 14 PRINCIPLES OF ISOLATION........................................................................................ 15 FACILITY ISOLATION OF COVID-19 CASES ............................................................ 16 HOME ISOLATION OF COVID-19 CASES ................................................................... 17 APPENDICES .................................................................................................................. -

Department of Road Transport and Safety Offices
DEPARTMENT OF ROAD TRANSPORT AND SAFETY OFFICES AND SERVICES MOLEPOLOLE • Registration & Licensing of vehicles and drivers • Driver Examination (Theory & Practical Tests) • Transport Inspectorate Tel: 5920148 Fax: 5910620 P/Bag 52 Molepolole Next to Molepolole Police MOCHUDI • Registration & Licensing of vehicles and drivers • Driver Examination (Theory & Practical Tests) • Transport Inspectorate P/Bag 36 Mochudi Tel : 5777127 Fax : 5748542 White House GABORONE Headquarters BBS Mall Plot no 53796 Tshomarelo House (Botswana Savings Bank) 1st, 2nd &3rd Floor Corner Lekgarapa/Letswai Road •Registration & Licensing of vehicles and drivers •Road safety (Public Education) Tel: 3688600/62 Fax : Fax: 3904067 P/Bag 0054 Gaborone GABORONE VTS – MARUAPULA • Registration & Licensing of vehicles and drivers • Driver Examination (Theory & Practical Tests) • Vehicle Examination Tel: 3912674/2259 P/Bag BR 318 B/Hurst Near Roads Training & Roads Maintenance behind Maruapula Flats GABORONE II – FAIRGROUNDS • Registration & Licensing of vehicles and drivers • Driver Examination : Theory Tel: 3190214/3911540/3911994 Fax : P/Bag 0054 Gaborone GABORONE - OLD SUPPLIES • Registration & Licensing of vehicles and drivers • Transport Permits • Transport Inspectorate Tel: 3905050 Fax :3932671 P/Bag 0054 Gaborone Plot 1221, Along Nkrumah Road, Near Botswana Power Corporation CHILDREN TRAFFIC SCHOOL •Road Safety Promotion for children only Tel: 3161851 P/Bag BR 318 B/Hurst RAMOTSWA •Registration & Licensing of vehicles and drivers •Driver Examination (Theory & Practical -

BOTSWANA July 2004 WHO Estimate of Number of People Requiring Treatment – End 2005: 60 000 Antiretroviral Therapy Target Declared by Country: 55 000 by 2005
BOTSWANA July 2004 WHO estimate of number of people requiring treatment – end 2005: 60 000 Antiretroviral therapy target declared by country: 55 000 by 2005 treat HIV sentinel surveillance Million Angola Zambia Kasane Livingstone in pregnant women Latest available year for 2001−2003 3by Percent seropositive 2005 Less than 1 1 − 4.9 Gweru Zimbabwe 5 − 9.9 Maun 10 − 14.9 Bulawayo 15 − 19.9 Francistown 20 − 29.9 Namibia Ghanzi 30 and more Gobabis Botswana Serowe Cities and towns Main roads Population density (pers./sq.Km) Less than 10 10 − 49 MolepololeMochudi Gaborone 50 − 99 Kanye 100 − 249 Lobatse 250 − 499 South Africa 500 − 749 Pretoria Tsabong 750 and more Johannesburg Map data sources: WHO/UNAIDS Epidemiological Fact Sheets and the United States Census Bureau Map production: WHO 0 62.5 125 250 Kilometers 1. Demographic and socioeconomic data 2. HIV indicators Date Estimate Source Date Estimate Source United 35.5 – WHO/ Total population (millions) 2004 1.8 Adult prevalence of HIV/AIDS (15–49 years) 2003 Nations 39.1% UNAIDS United Estimated number of people living with 330 000 – WHO/ Population in urban areas (%) 2003 51.3 2003 Nations HIV/AIDS (0–49 years) 380 000 UNAIDS WHO/ Life expectancy at birth (years) 2002 40.4 WHO Cumulative number of reported AIDS cases 2001 10 178 UNAIDS United Reported number of people receiving June Gross domestic product per capita (US$ ) 2001 2 872 18 000 WHO Nations antiretroviral therapy (15–49 years) 2004 Estimated total number needing antiretroviral WHO/ Government budget spent on health care (%) 2001 7.6 WHO 2003 60 000 therapy in 2005 UNAIDS HIV testing and counselling sites: number of not Per capita expenditure on health (US$) 2001 190 WHO sites available HIV testing and counselling sites: number of not Human Development Index 2001 0.614 UNDP people tested at all sites available Prevalence of HIV among adults with 2002 79% WHO 3. -
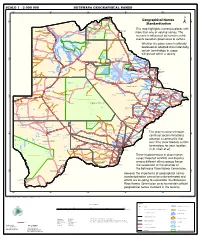
Geographical Names Standardization BOTSWANA GEOGRAPHICAL
SCALE 1 : 2 000 000 BOTSWANA GEOGRAPHICAL NAMES 20°0'0"E 22°0'0"E 24°0'0"E 26°0'0"E 28°0'0"E Kasane e ! ob Ch S Ngoma Bridge S " ! " 0 0 ' ' 0 0 ° Geographical Names ° ! 8 !( 8 1 ! 1 Parakarungu/ Kavimba ti Mbalakalungu ! ± n !( a Kakulwane Pan y K n Ga-Sekao/Kachikaubwe/Kachikabwe Standardization w e a L i/ n d d n o a y ba ! in m Shakawe Ngarange L ! zu ! !(Ghoha/Gcoha Gate we !(! Ng Samochema/Samochima Mpandamatenga/ This map highlights numerous places with Savute/Savuti Chobe National Park !(! Pandamatenga O Gudigwa te ! ! k Savu !( !( a ! v Nxamasere/Ncamasere a n a CHOBE DISTRICT more than one or varying names. The g Zweizwe Pan o an uiq !(! ag ! Sepupa/Sepopa Seronga M ! Savute Marsh Tsodilo !(! Gonutsuga/Gonitsuga scenario is influenced by human-centric Xau dum Nxauxau/Nxaunxau !(! ! Etsha 13 Jao! events based on governance or culture. achira Moan i e a h hw a k K g o n B Cakanaca/Xakanaka Mababe Ta ! u o N r o Moremi Wildlife Reserve Whether the place name is officially X a u ! G Gumare o d o l u OKAVANGO DELTA m m o e ! ti g Sankuyo o bestowed or adopted circumstantially, Qangwa g ! o !(! M Xaxaba/Cacaba B certain terminology in usage Nokaneng ! o r o Nxai National ! e Park n Shorobe a e k n will prevail within a society a Xaxa/Caecae/Xaixai m l e ! C u a n !( a d m a e a a b S c b K h i S " a " e a u T z 0 d ih n D 0 ' u ' m w NGAMILAND DISTRICT y ! Nxai Pan 0 m Tsokotshaa/Tsokatshaa 0 Gcwihabadu C T e Maun ° r ° h e ! 0 0 Ghwihaba/ ! a !( o 2 !( i ata Mmanxotae/Manxotae 2 g Botet N ! Gcwihaba e !( ! Nxharaga/Nxaraga !(! Maitengwe -

Mineralogy of Tailings Dump Around Selebi Phikwe Nickel-Copper Plant, Botswana
J. Appl. Sci. Environ. Mgt. 2004 JASEM ISSN 1119-8362 Available Online at All rights reserved http:// www.bioline.org.br/ja Vol. 8 (1) 37 - 44 Mineralogy of Tailings Dump around Selebi Phikwe Nickel-Copper Plant, Botswana 1*EKOSSE, G; 2VAN DEN HEEVER, D J; 3DE JAGER, L; 4TOTOLO, O 1*Department of Geology, University of Botswana, P/Bag 0022 Gaborone, Botswana 2VDH Industrial Hygiene CC.PO. Box 26792 Langenhoven Park, 9330, Free State, South Africa 3Department of Environmental Health, Technikon Free State, P/Bag X20539 Bloemfontein, Free State, South Africa 4Department of Environmental Science, University of Botswana, P/Bag 0022 Gaborone, Botswana ABSTRACT: This study aimed at mineralogically characterizing the tailings dump emanating from the mining and smelting of nickel-copper (Ni-Cu) at Selebi Phikwe, Botswana, Southern Africa. Samples of tailings dump around the Selebi Phikwe Ni-Cu plant were studied using petrographic microscopy and X-ray Powder Diffraction (XRPD) technique. The tailings dump comprised of the following minerals: nickelblodite, pyrrhotite, quartz, pentlandite, malachite, chalcopyrite, actinolite, cristobalite, tremolite, kaolinite, mica and albite. Nickelblodite and malachite were mineralized from the primary ore minerals in the tailings dump. The sulfur-rich minerals contained in the tailings dump serve as principal promoting sources for acid mine drainage in the immediate surrounding environment. Consequently, aspects of pollution management such as phytoremediation and phytomining, and environmental desulfurization could be introduced as pollution control measures at Selebi Phikwe. @JASEM Botswana is one of Africa's top three mineral some possible environmental implications resulting producers by value, including South Africa and the from the mining and extraction activities. -

Botswana Non-Profits
Botswana Non-Profits Note that other than the Botswana-UPenn Partnership, we do not endorse any of the following agencies. They are listed for information purposes only as non-profit organizations that may accept volunteers. Botswana-UPenn Partnership Location: Gaborone, Botswana Mission: To help build capacity in, clinical care, education, and research. Volunteer positions: clinical researchers Contact: Andrew Steenhoff, MD [email protected] Bana ba Keletso Orphan Day Care Centre Location: Molepolole, Botswana Mission: The broad based goal of the Centre is to support the children in the community who are orphaned. This involves providing them with the necessary skills, information, and training to reduce the psychological and social effects of being orphaned, and to reduce the risk of HIV/AIDS infection. The Centre's serves as a feeding centre, preschool and centre for school going children where they can receive counseling, as well as support for school work and mentorship. Volunteer positions: Contact: http://www.orfundfoundation.com/programs/ Elephants Without Borders Location: Kasane, Botswana Mission: Elephants Without Borders is a charitable organization dedicated to conserving wildlife and natural resources; through innovative research, education, and information sharing with all people. Volunteer positions: Contact: Michael Chase Phone/Fax: ++267 625-0202 New York State Affiliate office: EWB, Inc. 168 S. Marlin Rd., Grand Island, NY 14072, USA Phone: 001 (716) 773-1012 http://www.elephantswithoutborders.org/ Flying Mission Location: Gaborone, Botswana Mission: Flying mission serves by facilitating the spread of the Gospel of Jesus Christ through aviation, training and care ministries in Botswana, Zambia, and surrounding areas. Volunteer positions: Nurses / Fundraising / IT / Web design / Support / HR / Marketing etc. -
List of Schools Visited for Monitoring Visits
LIST OF SCHOOLS VISITED FOR MONITORING VISITS CENTRAL INSPECTORAL AREA LOCATION NAME OF SCHOOL MMADINARE Diloro Diloro MMADINARE Mmadinare Kelele MMADINARE Kgagodi Kgagodi MMADINARE Mmadinare Mmadinare MMADINARE Mmadinare Phethu Mphoeng MMADINARE Robelela Robelela MMADINARE Gojwane Sedibe MMADINARE Serule Serule MMADINARE Mmadinare Tlapalakoma BOTETI Rakops Etsile BOTETI Khumaga Khumaga BOTETI Khwee Khwee BOTETI Mopipi Manthabakwe BOTETI Mmadikola Mmadikola BOTETI Letlhakane Mokane BOTETI Mokoboxane Mokoboxane BOTETI Mokubilo Mokubilo BOTETI Moreomaoto Moreomaoto BOTETI Mosu Mosu BOTETI Motlopi Motlopi BOTETI Letlhakane Retlhatloleng Selibe Phikwe Selibe Phikwe Boitshoko Selibe Phikwe Selibe Phikwe Boswelakgomo Selibe Phikwe Selibe Phikwe Phikwe Selibe Phikwe Selibe Phikwe Tebogo BOBIRWA Bobonong Bobonong BOBIRWA Gobojango Gobojango BOBIRWA Bobonong Mabumahibidu BOBIRWA Bobonong Madikwe BOBIRWA Mogapi Mogapi BOBIRWA Molalatau Molalatau BOBIRWA Bobonong Rasetimela BOBIRWA Semolale Semolale BOBIRWA Tsetsebye Tsetsebye 1 | P a g e MAHALAPYE WEST Bonwapitse Bonwapitse MAHALAPYE WEST Mahalapye Leetile MAHALAPYE WEST Mokgenene Mokgenene MAHALAPYE WEST Moralane Moralane MAHALAPYE WEST Mosolotshane Mosolotshane MAHALAPYE WEST Otse Setlhamo MAHALAPYE WEST Mahalapye St James MAHALAPYE WEST Mahalapye Tshikinyega MHALAPYE EAST Mahalapye Flowertown MHALAPYE EAST Mahalapye Mahalapye MHALAPYE EAST Matlhako Matlhako MHALAPYE EAST Mmaphashalala Mmaphashalala MHALAPYE EAST Sefhare Mmutle PALAPYE NORTH Goo-Sekgweng Goo-Sekgweng PALAPYE NORTH Goo-Tau Goo-Tau -

Republic of Botswana
z. Öervenka REPUBLIC OF BOTSWANA A brief outline of its geographical setting, history, economy and policies The Scandinavian Institute of Afr{can Studies UPPSALA 1970 REPUBLIC OF BOTSWANA A brief outline of its geographical setting, history, economy and policies REPUBLIC OF BOTSWANA A br outline of s geographical setting, history, economy and pol ies by Zdenek Cervenka The and st Studi sala Nov 1970 Republic of Botswana CONTENTS page Territory l ~ Climate . 'il " ,;, " /I .. '" 1) q ... .. 'il l; t: " '" " .. ." ." C <II .. tI .. " .. o .. " t " • <l '" \I .. " .. (j .. " , 2 Popu lon",,, (I" "'''" .. (l"" (I"" •• 'il e·""" (I <>" '" jl, If •• Il .. " "" ... "." .... (I II 2 .~ ~ Language <'l II " III e II -Il iii " (> " .. e. •• (l .... " Q II " • l.I .. fl (I .. " .. " {I {I l) • Il " t 'il " '" t • 3 Pril1cipal tOWI1S Ot. iii " <l '11 • e " '" " • , • t1 t.> .. Il ........ , " ... (> .. " '" .. " ..... 'I' , 3 History Economy " iii " eJ (I • " , " .. .. (I ·t Q " • " " o " " ." /O ~ {I.." " .. " .. " .. .. (I .. • ID> • • • ~ ·e • .. .. 10 l)l'ess a.Dcl radio "Q" 11 l ..... c (I Il .. II CI " <) o " II> ........ Il .. Q \I Il 111 Il • '" '" 'Il .. " o e 13 Tirade Un.ions "... e Il 1'1 ... , <I ,. .. e .Q Q o "ll" , q t .. " .... Il 'il , " .... /I> $ .. " " ... o. 16 Political Parties , . 17 18 Local Government , ,., , . 19 20 1'1edical services .. ,. o •••••••••••••••••••••••••••••••• 22 External and external pol ies of Botswana 24 Diplomatic re sentation o •••••••••••••••••••••••• ,.. 28 l\ppend I. oC' Seretse M. Kharna, President of the Republic Botswana (short biography) endix II. Botswana a Highway (Statement by the Government of Botswana) lX III. Pinpoint Bl'· ~. a map Selected b iography Note: This essay was wr ten and c iled ent ly on the basis of publi cations and documents at the Scandinavian stitute of African Studies are listed the seleeted b iography. -

SABONET Report No 18
ii Quick Guide This book is divided into two sections: the first part provides descriptions of some common trees and shrubs of Botswana, and the second is the complete checklist. The scientific names of the families, genera, and species are arranged alphabetically. Vernacular names are also arranged alphabetically, starting with Setswana and followed by English. Setswana names are separated by a semi-colon from English names. A glossary at the end of the book defines botanical terms used in the text. Species that are listed in the Red Data List for Botswana are indicated by an ® preceding the name. The letters N, SW, and SE indicate the distribution of the species within Botswana according to the Flora zambesiaca geographical regions. Flora zambesiaca regions used in the checklist. Administrative District FZ geographical region Central District SE & N Chobe District N Ghanzi District SW Kgalagadi District SW Kgatleng District SE Kweneng District SW & SE Ngamiland District N North East District N South East District SE Southern District SW & SE N CHOBE DISTRICT NGAMILAND DISTRICT ZIMBABWE NAMIBIA NORTH EAST DISTRICT CENTRAL DISTRICT GHANZI DISTRICT KWENENG DISTRICT KGATLENG KGALAGADI DISTRICT DISTRICT SOUTHERN SOUTH EAST DISTRICT DISTRICT SOUTH AFRICA 0 Kilometres 400 i ii Trees of Botswana: names and distribution Moffat P. Setshogo & Fanie Venter iii Recommended citation format SETSHOGO, M.P. & VENTER, F. 2003. Trees of Botswana: names and distribution. Southern African Botanical Diversity Network Report No. 18. Pretoria. Produced by University of Botswana Herbarium Private Bag UB00704 Gaborone Tel: (267) 355 2602 Fax: (267) 318 5097 E-mail: [email protected] Published by Southern African Botanical Diversity Network (SABONET), c/o National Botanical Institute, Private Bag X101, 0001 Pretoria and University of Botswana Herbarium, Private Bag UB00704, Gaborone.