Terminal Addition, the Cambrian Radiation and the Phanerozoic Evolution of Bilaterian Form
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Conservation and Diversification of Appendage Identity Specification Mechanisms Along the Anteroposterior and Proximodistal Axes in Panarthropoda Frank W
University of Connecticut OpenCommons@UConn Doctoral Dissertations University of Connecticut Graduate School 8-23-2013 Conservation and Diversification of Appendage Identity Specification Mechanisms Along the Anteroposterior and Proximodistal Axes in Panarthropoda Frank W. Smith III University of Connecticut, [email protected] Follow this and additional works at: https://opencommons.uconn.edu/dissertations Recommended Citation Smith, Frank W. III, "Conservation and Diversification of Appendage Identity Specification Mechanisms Along the Anteroposterior and Proximodistal Axes in Panarthropoda" (2013). Doctoral Dissertations. 161. https://opencommons.uconn.edu/dissertations/161 Conservation and Diversification of Appendage Identity Specification Mechanisms Along the Anteroposterior and Proximodistal Axes in Panarthropoda Frank Wesley Smith III, PhD University of Connecticut, 2013 A key characteristic of arthropods is their diverse serially homologous segmented appendages. This dissertation explores diversification of these appendages, and the developmental mechanisms producing them. In Chapter 1, the roles of genes that specify antennal identity in Drosophila melanogaster were investigated in the flour beetle Tribolium castaneum. Antenna-to-leg transformations occurred in response to RNA interference (RNAi) against homothorax, extradenticle, spineless and Distal-less. However, for homothorax/extradenticle RNAi, the extent of transformation along the proximodistal axis differed between embryogenesis and metamorphosis. This suggests that distinct mechanisms specify antennal identity during flour beetle embryogenesis and metamorphosis and leads to a model for the evolution of the Drosophila antennal identity mechanism. Homothorax/Extradenticle acquire many of their identity specification roles by acting as Hox cofactors. In chapter 2, the metamorphic roles of the Hox genes, extradenticle, and homothorax were compared in T. castaneum. homothorax/extradenticle RNAi and Hox RNAi produced similar body wall phenotypes but different appendage phenotypes. -

Brains of Primitive Chordates 439
Brains of Primitive Chordates 439 Brains of Primitive Chordates J C Glover, University of Oslo, Oslo, Norway although providing a more direct link to the evolu- B Fritzsch, Creighton University, Omaha, NE, USA tionary clock, is nevertheless hampered by differing ã 2009 Elsevier Ltd. All rights reserved. rates of evolution, both among species and among genes, and a still largely deficient fossil record. Until recently, it was widely accepted, both on morpholog- ical and molecular grounds, that cephalochordates Introduction and craniates were sister taxons, with urochordates Craniates (which include the sister taxa vertebrata being more distant craniate relatives and with hemi- and hyperotreti, or hagfishes) represent the most chordates being more closely related to echinoderms complex organisms in the chordate phylum, particu- (Figure 1(a)). The molecular data only weakly sup- larly with respect to the organization and function of ported a coherent chordate taxon, however, indicat- the central nervous system. How brain complexity ing that apparent morphological similarities among has arisen during evolution is one of the most chordates are imposed on deep divisions among the fascinating questions facing modern science, and it extant deuterostome taxa. Recent analysis of a sub- speaks directly to the more philosophical question stantially larger number of genes has reversed the of what makes us human. Considerable interest has positions of cephalochordates and urochordates, pro- therefore been directed toward understanding the moting the latter to the most closely related craniate genetic and developmental underpinnings of nervous relatives (Figure 1(b)). system organization in our more ‘primitive’ chordate relatives, in the search for the origins of the vertebrate Comparative Appearance of Brains, brain in a common chordate ancestor. -

Transformations of Lamarckism Vienna Series in Theoretical Biology Gerd B
Transformations of Lamarckism Vienna Series in Theoretical Biology Gerd B. M ü ller, G ü nter P. Wagner, and Werner Callebaut, editors The Evolution of Cognition , edited by Cecilia Heyes and Ludwig Huber, 2000 Origination of Organismal Form: Beyond the Gene in Development and Evolutionary Biology , edited by Gerd B. M ü ller and Stuart A. Newman, 2003 Environment, Development, and Evolution: Toward a Synthesis , edited by Brian K. Hall, Roy D. Pearson, and Gerd B. M ü ller, 2004 Evolution of Communication Systems: A Comparative Approach , edited by D. Kimbrough Oller and Ulrike Griebel, 2004 Modularity: Understanding the Development and Evolution of Natural Complex Systems , edited by Werner Callebaut and Diego Rasskin-Gutman, 2005 Compositional Evolution: The Impact of Sex, Symbiosis, and Modularity on the Gradualist Framework of Evolution , by Richard A. Watson, 2006 Biological Emergences: Evolution by Natural Experiment , by Robert G. B. Reid, 2007 Modeling Biology: Structure, Behaviors, Evolution , edited by Manfred D. Laubichler and Gerd B. M ü ller, 2007 Evolution of Communicative Flexibility: Complexity, Creativity, and Adaptability in Human and Animal Communication , edited by Kimbrough D. Oller and Ulrike Griebel, 2008 Functions in Biological and Artifi cial Worlds: Comparative Philosophical Perspectives , edited by Ulrich Krohs and Peter Kroes, 2009 Cognitive Biology: Evolutionary and Developmental Perspectives on Mind, Brain, and Behavior , edited by Luca Tommasi, Mary A. Peterson, and Lynn Nadel, 2009 Innovation in Cultural Systems: Contributions from Evolutionary Anthropology , edited by Michael J. O ’ Brien and Stephen J. Shennan, 2010 The Major Transitions in Evolution Revisited , edited by Brett Calcott and Kim Sterelny, 2011 Transformations of Lamarckism: From Subtle Fluids to Molecular Biology , edited by Snait B. -

Evolution by Natural Selection, Formulated Independently by Charles Darwin and Alfred Russel Wallace
UNIT 4 EVOLUTIONARY PATT EVOLUTIONARY E RNS AND PROC E SS E Evolution by Natural S 22 Selection Natural selection In this chapter you will learn that explains how Evolution is one of the most populations become important ideas in modern biology well suited to their environments over time. The shape and by reviewing by asking by applying coloration of leafy sea The rise of What is the evidence for evolution? Evolution in action: dragons (a fish closely evolutionary thought two case studies related to seahorses) 22.1 22.4 are heritable traits that with regard to help them to hide from predators. The pattern of evolution: The process of species have changed evolution by natural and are related 22.2 selection 22.3 keeping in mind Common myths about natural selection and adaptation 22.5 his chapter is about one of the great ideas in science: the theory of evolution by natural selection, formulated independently by Charles Darwin and Alfred Russel Wallace. The theory explains how T populations—individuals of the same species that live in the same area at the same time—have come to be adapted to environments ranging from arctic tundra to tropical wet forest. It revealed one of the five key attributes of life: Populations of organisms evolve. In other words, the heritable characteris- This chapter is part of the tics of populations change over time (Chapter 1). Big Picture. See how on Evolution by natural selection is one of the best supported and most important theories in the history pages 516–517. of scientific research. -

Many but Not All Lineage-Specific Genes Can Be Explained by Homology Detection Failure
bioRxiv preprint doi: https://doi.org/10.1101/2020.02.27.968420; this version posted April 14, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Many but not all lineage-specific genes can be explained by homology detection failure Caroline M. Weisman1, Andrew W. Murray1, Sean R. Eddy1,2,3 1 Department of Molecular & Cellular Biology, 2 Howard Hughes Medical Institute, 3 John A. Paulson School of Engineering and Applied Sciences, Harvard University, Cambridge MA, USA Abstract Genes for which homologs can be detected only in a limited group of evolutionarily related species, called “lineage-specific genes,” are pervasive: essentially every lineage has them, and they often comprise a sizable fraction of the group’s total genes. Lineage-specific genes are often interpreted as “novel” genes, representing genetic novelty born anew within that lineage. Here, we develop a simple method to test an alternative null hypothesis: that lineage-specific genes do have homologs outside of the lineage that, even while evolving at a constant rate in a novelty-free manner, have merely become undetectable by search algorithms used to infer homology. We show that this null hypothesis is sufficient to explain the lack of detected homologs of a large number of lineage-specific genes in fungi and insects. However, we also find that a minority of lineage-specific genes in both clades are not well-explained by this novelty- free model. -

The Origins of Chordate Larvae Donald I Williamson* Marine Biology, University of Liverpool, Liverpool L69 7ZB, United Kingdom
lopmen ve ta e l B Williamson, Cell Dev Biol 2012, 1:1 D io & l l o l g DOI: 10.4172/2168-9296.1000101 e y C Cell & Developmental Biology ISSN: 2168-9296 Research Article Open Access The Origins of Chordate Larvae Donald I Williamson* Marine Biology, University of Liverpool, Liverpool L69 7ZB, United Kingdom Abstract The larval transfer hypothesis states that larvae originated as adults in other taxa and their genomes were transferred by hybridization. It contests the view that larvae and corresponding adults evolved from common ancestors. The present paper reviews the life histories of chordates, and it interprets them in terms of the larval transfer hypothesis. It is the first paper to apply the hypothesis to craniates. I claim that the larvae of tunicates were acquired from adult larvaceans, the larvae of lampreys from adult cephalochordates, the larvae of lungfishes from adult craniate tadpoles, and the larvae of ray-finned fishes from other ray-finned fishes in different families. The occurrence of larvae in some fishes and their absence in others is correlated with reproductive behavior. Adult amphibians evolved from adult fishes, but larval amphibians did not evolve from either adult or larval fishes. I submit that [1] early amphibians had no larvae and that several families of urodeles and one subfamily of anurans have retained direct development, [2] the tadpole larvae of anurans and urodeles were acquired separately from different Mesozoic adult tadpoles, and [3] the post-tadpole larvae of salamanders were acquired from adults of other urodeles. Reptiles, birds and mammals probably evolved from amphibians that never acquired larvae. -

Abstract Introduction
Marine and Freshwater Miscellanea III KEY TRAITS OF AMPHIOXUS SPECIES (CEPHALOCHORDATA) AND THE GOLT1 Daniel Pauly and Elaine Chu Sea Around Us, Institute for the Oceans and Fisheries University of British Columbia, Vancouver, B.C, Canada Email: [email protected] Abstract Major biological traits of amphioxus species (Cephalochordata) are presented with emphasis on the size reached by their 32 valid species in the genera Asymmetron (2 spp.), Branchiostoma (25 spp.), and Epigonichthys (5 spp.) and on related features, i.e., growth parameters and size at first maturity. Overall, these traits combined with features of their respiration, suggest that the cephalochordates conform to the Gill Oxygen Limitation Theory (GOLT), which relates the growth performance of water-breathing ectotherms to the surface area of their respiratory organ(s). Introduction The small fish-like animals know as ‘lancelet‘ or ‘amphioxius’ belong the subphylum Cephalochordata, which is either a sister group, or related to the ancestor of the vertebrate animals (see Garcia-Fernàndez and Benito-Gutierrez 2008). The cephalochordates consist of 3 families (the Asymmetronidae, Epigonichthyidae and Branchiostomidae), with one genus each, Asymmetron (2 spp.), Branchiostoma (24 spp.) and Epigonichthys (6 spp.), as detailed in Table 1 and SeaLifeBase (www.sealifebase.org). This contribution is to assemble some of the basic biological traits of lancelets (Figure 1), notably the maximum size each of their 34 species can reach, which is easily their most important attribute, though it is often ignored (Haldane 1926). Finally, reported lengths at first maturity of cephalochordates were related to the corresponding, population-specific maximum length, to test whether these animals mature as predicted by the Gill- Oxygen Limitation Theory (GOLT; see Pauly 2021a, 2021b). -

Constraints on the Timescale of Animal Evolutionary History
Palaeontologia Electronica palaeo-electronica.org Constraints on the timescale of animal evolutionary history Michael J. Benton, Philip C.J. Donoghue, Robert J. Asher, Matt Friedman, Thomas J. Near, and Jakob Vinther ABSTRACT Dating the tree of life is a core endeavor in evolutionary biology. Rates of evolution are fundamental to nearly every evolutionary model and process. Rates need dates. There is much debate on the most appropriate and reasonable ways in which to date the tree of life, and recent work has highlighted some confusions and complexities that can be avoided. Whether phylogenetic trees are dated after they have been estab- lished, or as part of the process of tree finding, practitioners need to know which cali- brations to use. We emphasize the importance of identifying crown (not stem) fossils, levels of confidence in their attribution to the crown, current chronostratigraphic preci- sion, the primacy of the host geological formation and asymmetric confidence intervals. Here we present calibrations for 88 key nodes across the phylogeny of animals, rang- ing from the root of Metazoa to the last common ancestor of Homo sapiens. Close attention to detail is constantly required: for example, the classic bird-mammal date (base of crown Amniota) has often been given as 310-315 Ma; the 2014 international time scale indicates a minimum age of 318 Ma. Michael J. Benton. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Philip C.J. Donoghue. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Robert J. -

Stages of Embryonic Development of the Zebrafish
DEVELOPMENTAL DYNAMICS 2032553’10 (1995) Stages of Embryonic Development of the Zebrafish CHARLES B. KIMMEL, WILLIAM W. BALLARD, SETH R. KIMMEL, BONNIE ULLMANN, AND THOMAS F. SCHILLING Institute of Neuroscience, University of Oregon, Eugene, Oregon 97403-1254 (C.B.K., S.R.K., B.U., T.F.S.); Department of Biology, Dartmouth College, Hanover, NH 03755 (W.W.B.) ABSTRACT We describe a series of stages for Segmentation Period (10-24 h) 274 development of the embryo of the zebrafish, Danio (Brachydanio) rerio. We define seven broad peri- Pharyngula Period (24-48 h) 285 ods of embryogenesis-the zygote, cleavage, blas- Hatching Period (48-72 h) 298 tula, gastrula, segmentation, pharyngula, and hatching periods. These divisions highlight the Early Larval Period 303 changing spectrum of major developmental pro- Acknowledgments 303 cesses that occur during the first 3 days after fer- tilization, and we review some of what is known Glossary 303 about morphogenesis and other significant events that occur during each of the periods. Stages sub- References 309 divide the periods. Stages are named, not num- INTRODUCTION bered as in most other series, providing for flexi- A staging series is a tool that provides accuracy in bility and continued evolution of the staging series developmental studies. This is because different em- as we learn more about development in this spe- bryos, even together within a single clutch, develop at cies. The stages, and their names, are based on slightly different rates. We have seen asynchrony ap- morphological features, generally readily identi- pearing in the development of zebrafish, Danio fied by examination of the live embryo with the (Brachydanio) rerio, embryos fertilized simultaneously dissecting stereomicroscope. -
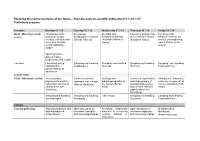
Exploring the Marine Meiofauna of the Azores – from Discovery to Scientific Publication (15.7.-24.7.19) Preliminary Program
Exploring the marine meiofauna of the Azores – from discovery to scientific publication (15.7.-24.7.19) Preliminary program: Schedule Monday 15.7.19 Tuesday 16.7.19 Wednesday 17.7.19 Thursday 18.7.19 Friday 19.7.19 09.00 - Morning session Introduction to the Meiofaunal Intertidal and Taxonomy and diversity The Proseriata Lecture workshop (people, Scalidophora (Andreas meiofaunal annelids of meiofaunal molluscs (Platyhelminthes): the facilities, schedule and Schmidt-Rhaesa) (Alejandro Martínez (Katharina Jörger) interest of unappealing aims; Ana Ricardo Garcia) worms (Marco Curini- Costa/ Katharina Galetti) Jörger) Opening lecture: Azores marine biodiversity (Ana Costa) Lab work Laboratory set-up, Sampling and handling Sampling and handling Sampling and handling Sampling and handling handling and Scalidophora Annelida Mollusca Platyhelminthes documentation of specimens LUNCH TIME 14.00 - Afternoon session Acoelomorpha – Marine nematodes: Geology and Taxonomy, systematics Rhabdocoel flatworms, phylogenetic position, taxonomy and ecology paleobiogeography of and biogeography of a diverse component of systematic and how to (Alberto Navarrete) the Azores (Sérgio meiofaunal Nemertea marine meiofauna (Tom identify them (Ulf Avila) and relevant research Artois) Jondelius) opportunities (Jon Norenburg) Sampling and handling Sampling and handling Lab session Sampling and handling Sampling and handling Acoelomorpha Nematoda Nemertea Platyhelminthes DINNER Evening gathering Sampling strategies and Open discussion on Adressing biodiversity Open discussion -

Introduction to the Body-Plan of Onychophora and Tardigrada
Joakim Eriksson, 02.12.13 Animal bodyplans Onychophora Tardigrada Bauplan, bodyplan • A Bauplan is a set of conservative characters that are typical for one group but distinctively different from a Bauplan of another group Arthropoda Bauplan 2 Bauplan 3 Bauplan 4 Tardigrada Onychophora Euarthropoda/Arthropoda Insects Chelicerates Myriapods Crustaceans Arthropoda/Panarthropoda Bauplan 1 Arthropod characters • Body segmented, with limbs on several segments • Adult body cavity a haemocoel that extends into the limbs • Cuticle of α-chitin which is molted regularly • Appendages with chitinous claws, and mixocoel with metanephridia and ostiate heart (absent in tardigrades) • Engrailed gene expressed in posterior ectoderm of each segment • Primitively possess a terminal mouth, a non-retractable proboscis, and a thick integument of diverse plates Phylogenomics and miRNAs suggest velvets worm are the sister group to the arthropods within a monophyletic Panarthropoda. Campbell L I et al. PNAS 2011;108:15920-15924 Fossil arthropods, the Cambrian explosion Aysheaia An onychophoran or phylum of its own? Anomalocaris A phylum on its own? Crown group and stem group Arthropoda Bauplan 2 Bauplan 3 Bauplan 4 Tardigrada Onychophora Euarthropoda/Arthropoda Arthropoda/Panarthropoda Bauplan 1 How do arthropods relate to other animal groups Articulata Georges Cuvier, 1817 Characters uniting articulata: •Segmentation •Ventral nerv cord •Teloblastic growth zone Ecdysozoa Aguinaldo et al 1997 Ecdysozoa Character uniting ecdysozoa: •Body covered by cuticle of α-chitin -

Metamerism in Cephalochordates and the Problem of the Vertebrate Head TAKAYUKI ONAI*,1,2, NORITAKA ADACHI1 and SHIGERU KURATANI1
Int. J. Dev. Biol. 61: 621-632 (2017) doi: 10.1387/ijdb.170121to www.intjdevbiol.com Metamerism in cephalochordates and the problem of the vertebrate head TAKAYUKI ONAI*,1,2, NORITAKA ADACHI1 and SHIGERU KURATANI1 1Evolutionary Morphology Laboratory, RIKEN center for Developmental Biology, Chuo-ku, Kobe, Japan and 2Department of Anatomy, School of Medical Sciences, University of Fukui, Matsuokashimoaizuki, Japan ABSTRACT The vertebrate head characteristically exhibits a complex pattern with sense organs, brain, paired eyes and jaw muscles, and the brain case is not found in other chordates. How the extant vertebrate head has evolved remains enigmatic. Historically, there have been two conflicting views on the origin of the vertebrate head, segmental and non-segmental views. According to the segmentalists, the vertebrate head is organized as a metameric structure composed of segments equivalent to those in the trunk; a metamere in the vertebrate head was assumed to consist of a somite, a branchial arch and a set of cranial nerves, considering that the head evolved from rostral segments of amphioxus-like ancestral vertebrates. Non-segmentalists, however, considered that the vertebrate head was not segmental. In that case, the ancestral state of the vertebrate head may be non-segmented, and rostral segments in amphioxus might have been secondarily gained, or extant vertebrates might have evolved through radical modifications of amphioxus-like ancestral vertebrate head. Comparative studies of mesodermal development in amphioxus and vertebrate gastrula embryos have revealed that mesodermal gene expressions become segregated into two domains anteroposteriorly to specify the head mesoderm and trunk mesoderm only in vertebrates; in this segregation, key genes such as delta and hairy, involved in segment formation, are expressed in the trunk mesoderm, but not in the head mesoderm, strongly suggesting that the head meso- derm of extant vertebrates is not segmented.