Reproductive Behavior Drives Female Space Use in a Sedentary Neotropical Frog
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

HOME RANGE OVERLAP and SPATIAL-TEMPORAL INTERACTIONS in FEMALE FISHERS on the HOOPA INDIAN RESERVATION in CALIFORNIA by Kerry M
HOME RANGE OVERLAP AND SPATIAL-TEMPORAL INTERACTIONS IN FEMALE FISHERS ON THE HOOPA INDIAN RESERVATION IN CALIFORNIA By Kerry M. Rennie A Thesis Presented to The Faculty of Humboldt State University In Partial Fulfillment of the Requirements for the Degree Master of Science in Natural Resources: Wildlife Committee Membership Dr. Micaela Szykman Gunther, Committee Chair J. Mark Higley, Committee Member Dr. Matthew Johnson, Committee Member Dr. William J. Zielinski, Committee Member Dr. Yvonne Everett, Graduate Coordinator July 2015 ABSTRACT HOME RANGE OVERLAP AND SPATIAL-TEMPORAL INTERACTIONS IN FEMALE FISHERS ON THE HOOPA INDIAN RESERVATION Kerry M. Rennie Spatial organization and interactions within animal populations can have important effects on population demography, social dynamics, and genetic composition. Understanding social behavior and relatedness of individuals in a population can advise species management and conservation. Previous research suggests that fishers (Pekania pennanti) are asocial and exhibit intrasexual territoriality, where home ranges rarely overlap with the same sex. On the Hoopa Valley Indian Reservation in California, female fishers appear to overlap with members of the same sex to a greater extent than most studies report. I tested the hypothesis that female fishers tolerate some degree of spatial overlap, but that co- occurrence in the area of overlap is uncommon. I also tested the hypothesis that this overlap is mediated by habitat selection and female philopatry. I found that female fishers share a large proportion of their home ranges and overlap at a high frequency. However, dynamic interactions revealed fishers avoided each other in both space and time. Habitat used in overlap areas differed from what was available to them in non-overlapping areas but lacked a consistent pattern of ii selection among habitat types. -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -
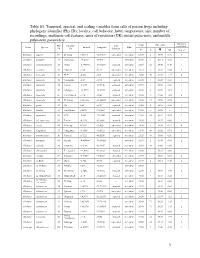
1 Table S1. Temporal, Spectral, and Scaling Variables from Calls Of
Table S1. Temporal, spectral, and scaling variables from calls of poison frogs including phylogeny identifier (Phy ID), locality, call behavior, habit, temperature, size, number of recordings, multinote call features, units of repetition (UR), initial pulse-note, and middle pulse-note parameters. Analyzed Phy Locality Call Temp SVL (mm) Genus Species Latitude Longitude Habit recordings ID ID Behavior °C N ! SD N of ♂ Allobates algorei 60 El Tama 7.65375 -72.19137 concealed terrestrial 23.50 8 18.90 0.70 3 Allobates brunneus 37 Guimaraes -15.2667 -55.5311 -- terrestrial 26.50 1 16.13 0.00 1 Allobates caeruleodactylus 48 Borba -4.398593 -59.60251 exposed terrestrial 25.60 12 15.50 0.40 1 Allobates crombiei 52 Altamira -3.65 -52.38 concealed terrestrial 24.10 2 18.10 0.04 2 Allobates femoralis 43 ECY -0.633 -76.5 concealed terrestrial 25.60 20 23.58 1.27 6 Allobates femoralis 46 Porongaba -8.67 -72.78 exposed terrestrial 25.00 1 25.38 0.00 1 Allobates femoralis 44 Leticia -4.2153 -69.9406 exposed terrestrial 25.50 1 20.90 0.00 1 Allobates femoralis 40 Albergue -12.8773 -71.3865 exposed terrestrial 26.00 6 21.98 2.18 1 Allobates femoralis 41 CAmazonico -12.6 -70.08 exposed terrestrial 26.00 12 22.43 1.06 4 Allobates femoralis 45 El Palmar 8.333333 -61.66667 concealed terrestrial 24.00 27 25.50 0.76 1 Allobates granti 49 FG 3.62 -53.17 exposed terrestrial 24.60 8 16.15 0.55 1 Allobates humilis 59 San Ramon 8.8678 -70.4861 concealed terrestrial 19.50 -- 21.80 -- 1 Allobates insperatus 54 ECY -0.633 -76.4005 exposed terrestrial 24.60 18 16.64 0.93 7 Allobates aff. -

New Behavioral Insights Into Home Range Orientation of the House Mouse (Mus Musculus) by Blythe E
New Behavioral Insights Into Home Range Orientation of the House Mouse (Mus musculus) By Blythe E. Alexander Submitted to the graduate degree program in Ecology and Evolutionary Biology and the Graduate Faculty of the University of Kansas in partial fulfillment of the requirements for the degree of Doctor of Philosophy. ________________________________ Rudolf Jander ________________________________ Helen M. Alexander ________________________________ Kenneth B. Armitage ________________________________ Linda Trueb ________________________________ Stephen Egbert Date Defended: May 4, 2011 The Dissertation Committee for Blythe Alexander certifies that this is the approved version of the following dissertation: New Behavioral Insights Into Home Range Orientation of the House Mouse (Mus musculus) ________________________________ Rudolf Jander Date approved: ii Abstract Home‐range orientation is a necessity for an animal that maintains an area of daily activity. The ability to navigate efficiently among goals not perceived at the starting point requires the animal to rely on place recognition and vector knowledge. These two components of navigation allow the animal to dynamically update its current position and link that position with the locomotor distance and direction needed to reach a goal. In order to use place knowledge and vector knowledge the animal must learn and remember relevant spatial information obtained from the environment and from internal feedback. The research in this dissertation focuses on behavioral components of topographic orientation, using the house mouse as a model species. Specifically, this research made important discoveries in three main areas: 1) locomotor exploration behavior, 2) the use of learned spatial information for compass orientation, and 3) testable hypotheses based on the controversial cognitive map. In Chapter 1, I used a radial arm maze to find a systematic locomotor component to exploration behavior, which is typically described as random movement. -

A Rapid Biological Assessment of the Upper Palumeu River Watershed (Grensgebergte and Kasikasima) of Southeastern Suriname
Rapid Assessment Program A Rapid Biological Assessment of the Upper Palumeu River Watershed (Grensgebergte and Kasikasima) of Southeastern Suriname Editors: Leeanne E. Alonso and Trond H. Larsen 67 CONSERVATION INTERNATIONAL - SURINAME CONSERVATION INTERNATIONAL GLOBAL WILDLIFE CONSERVATION ANTON DE KOM UNIVERSITY OF SURINAME THE SURINAME FOREST SERVICE (LBB) NATURE CONSERVATION DIVISION (NB) FOUNDATION FOR FOREST MANAGEMENT AND PRODUCTION CONTROL (SBB) SURINAME CONSERVATION FOUNDATION THE HARBERS FAMILY FOUNDATION Rapid Assessment Program A Rapid Biological Assessment of the Upper Palumeu River Watershed RAP (Grensgebergte and Kasikasima) of Southeastern Suriname Bulletin of Biological Assessment 67 Editors: Leeanne E. Alonso and Trond H. Larsen CONSERVATION INTERNATIONAL - SURINAME CONSERVATION INTERNATIONAL GLOBAL WILDLIFE CONSERVATION ANTON DE KOM UNIVERSITY OF SURINAME THE SURINAME FOREST SERVICE (LBB) NATURE CONSERVATION DIVISION (NB) FOUNDATION FOR FOREST MANAGEMENT AND PRODUCTION CONTROL (SBB) SURINAME CONSERVATION FOUNDATION THE HARBERS FAMILY FOUNDATION The RAP Bulletin of Biological Assessment is published by: Conservation International 2011 Crystal Drive, Suite 500 Arlington, VA USA 22202 Tel : +1 703-341-2400 www.conservation.org Cover photos: The RAP team surveyed the Grensgebergte Mountains and Upper Palumeu Watershed, as well as the Middle Palumeu River and Kasikasima Mountains visible here. Freshwater resources originating here are vital for all of Suriname. (T. Larsen) Glass frogs (Hyalinobatrachium cf. taylori) lay their -

Ecology and Evolution of Phytotelm- Jreeding Anurans
* ECOLOGY AND EVOLUTION OF PHYTOTELM- JREEDING ANURANS Richard M. Lehtinen Editor MISCELLANEOUS PUBLICATIONS I--- - MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN, NO. 193 Ann Ahr, November, 2004 PUBLICATIONS OF THE MUSEUM OF ZQOLOGY, UNIVERSITY OF MICHIGAN NO. 192 J. B. BURCII,Editot* Ku1.1: SI.EFANOAND JANICEPAPPAS, Assistant Editoras The publications of the Museum of Zoology, The University of Michigan, consist primarily of two series-the Miscellaneous P~rhlicationsand the Occasional Papers. Both serics were founded by Dr. Bryant Walker, Mr. Bradshaw H. Swales, and Dr. W. W. Newcomb. Occasionally the Museum publishes contributions outside of thesc series; beginning in 1990 these are titled Special Publications and are numbered. All s~tbmitledmanuscripts to any of the Museum's publications receive external review. The Occasiontrl Papers, begun in 1913, sellie as a mcdium for original studies based prii~cipallyupon the collections in the Museum. They are issued separately. When a sufficient number of pages has been printed to make a volume, a title page, table of contents, and an index are supplied to libraries and individuals on the mailing list for the series. The Mi.scelluneous Puhlicutions, initiated in 1916, include monographic studies, papers on field and museum techniques, and other contributions not within the scope of the Occasional Papers, and are publislled separately. It is not intended that they bc grouped into volumes. Each number has a title page and, when necessary, a table of contents. A complete list of publications on Mammals, Birds, Reptiles and Amphibians, Fishes, Insects, Mollusks, and other topics is avail- able. Address inquiries to Publications, Museum of Zoology, The University of Michigan, Ann Arbor, Michigan 48 109-1079. -

Home Range Estimates, Variability, and Sample Size Marc Bekoff University of Colorado
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln USGS Northern Prairie Wildlife Research Center Wildlife Damage Management, Internet Center for 1984 Simulation analyses of space use: Home range estimates, variability, and sample size Marc Bekoff University of Colorado L. David Mech USGS Northern Prairie Wildlife Research Center, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/usgsnpwrc Part of the Animal Sciences Commons, Behavior and Ethology Commons, Biodiversity Commons, Environmental Policy Commons, Recreation, Parks and Tourism Administration Commons, and the Terrestrial and Aquatic Ecology Commons Bekoff, Marc and Mech, L. David, "Simulation analyses of space use: Home range estimates, variability, and sample size" (1984). USGS Northern Prairie Wildlife Research Center. 371. https://digitalcommons.unl.edu/usgsnpwrc/371 This Article is brought to you for free and open access by the Wildlife Damage Management, Internet Center for at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in USGS Northern Prairie Wildlife Research Center by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Behavior Research Methods, Instruments, & Computers 1984, Vol. 16 (1), 32-37 COMPUTER SIMULATION Simulation analyses of space use: Home range estimates, variability, and sample size MARC BEKOFF University ofColorado, Boulder, Colorado and L. DAVID MECH Patuxent Wildlife Research Center, U. SFish and Wildlife Service, Laurel, Maryland Simulations of space use by animals were run to determine the relationship among home range area estimates, variability, and sample size (number of locations). As sample size in creased, home range size increased asymptotically, whereas variability decreased among mean home range area estimates generated by multiple simulations for the same sample size. -

Taxonomic Checklist of Amphibian Species Listed in the CITES
CoP17 Doc. 81.1 Annex 5 (English only / Únicamente en inglés / Seulement en anglais) Taxonomic Checklist of Amphibian Species listed in the CITES Appendices and the Annexes of EC Regulation 338/97 Species information extracted from FROST, D. R. (2015) "Amphibian Species of the World, an online Reference" V. 6.0 (as of May 2015) Copyright © 1998-2015, Darrel Frost and TheAmericanMuseum of Natural History. All Rights Reserved. Additional comments included by the Nomenclature Specialist of the CITES Animals Committee (indicated by "NC comment") Reproduction for commercial purposes prohibited. CoP17 Doc. 81.1 Annex 5 - p. 1 Amphibian Species covered by this Checklist listed by listed by CITES EC- as well as Family Species Regulation EC 338/97 Regulation only 338/97 ANURA Aromobatidae Allobates femoralis X Aromobatidae Allobates hodli X Aromobatidae Allobates myersi X Aromobatidae Allobates zaparo X Aromobatidae Anomaloglossus rufulus X Bufonidae Altiphrynoides malcolmi X Bufonidae Altiphrynoides osgoodi X Bufonidae Amietophrynus channingi X Bufonidae Amietophrynus superciliaris X Bufonidae Atelopus zeteki X Bufonidae Incilius periglenes X Bufonidae Nectophrynoides asperginis X Bufonidae Nectophrynoides cryptus X Bufonidae Nectophrynoides frontierei X Bufonidae Nectophrynoides laevis X Bufonidae Nectophrynoides laticeps X Bufonidae Nectophrynoides minutus X Bufonidae Nectophrynoides paulae X Bufonidae Nectophrynoides poyntoni X Bufonidae Nectophrynoides pseudotornieri X Bufonidae Nectophrynoides tornieri X Bufonidae Nectophrynoides vestergaardi -

Anura: Aromobatidae) from the Type Locality (Cachoeira Do Espelho), Xingu River, Brazil
Zootaxa 3475: 86–88 (2012) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2012 · Magnolia Press Correspondence ISSN 1175-5334 (online edition) urn:lsid:zoobank.org:pub:D975CEA0-9884-4AF6-BB33-73B76983BABD Advertisement call and colour in life of Allobates crombiei (Morales) “2000” [2002] (Anura: Aromobatidae) from the type Locality (Cachoeira do Espelho), Xingu River, Brazil ALBERTINA P. LIMA1, LUCIANA K. ERDTMANN1 & ADOLFO AMÉZQUITA2 1Coordenação de Biodiversidade, Instituto Nacional de Pesquisas da Amazônia Av. André Araújo, 2936, 69083-000, Manaus, AM, Brasil 2Department of Biological Sciences, Universidad de los Andes, AA 4976, Bogotá, Colombia 1Corresponding author: E-mail: [email protected] Allobates crombiei was described by Morales, “2000” [2002] based on specimens collected by Ronald I. Crombie from Cachoeira do Espelho, on the right bank of the Xingu River, Pará State, Brazil. The original description was short and did not include the call or colour in life. Rodrigues & Caramaschi (2004) suggested that the taxonomic status of this species need be clarified. We are confident that the species collected and recorded by us is Allobates crombiei (Morales) “2000” [2002] because this is the only species of Allobates found calling in forest near Cachoeira do Espelho, and the character diagnosis in preserved specimens is similar, except that, based on preserved specimens, Morales (2002) considered the ventrolateral and the oblique lateral stripes to be absent. This may be because they are imperceptible in preserved specimens. However, unlike recent authors, Morales (2002) also considered the oblique lateral stripe to be absent in Allobates brunneus, Allobates gasconi and Allobates ornatus, in which he illustrated diffuse spots. -

Animal Umwelten in a Changing World
Tartu Semiotics Library 18 Tartu Tartu Semiotics Library 18 Animal umwelten in a changing world: Zoosemiotic perspectives represents a clear and concise review of zoosemiotics, present- ing theories, models and methods, and providing interesting examples of human–animal interactions. The reader is invited to explore the umwelten of animals in a successful attempt to retrieve the relationship of people with animals: a cornerstone of the past common evolutionary processes. The twelve chapters, which cover recent developments in zoosemiotics and much more, inspire the reader to think about the human condition and about ways to recover our lost contact with the animal world. Written in a clear, concise style, this collection of articles creates a wonderful bridge between Timo Maran, Morten Tønnessen, human and animal worlds. It represents a holistic approach Kristin Armstrong Oma, rich with suggestions for how to educate people to face the dynamic relationships with nature within the conceptual Laura Kiiroja, Riin Magnus, framework of the umwelt, providing stimulus and opportuni- Nelly Mäekivi, Silver Rattasepp, ties to develop new studies in zoosemiotics. Professor Almo Farina, CHANGING WORLD A IN UMWELTEN ANIMAL Paul Thibault, Kadri Tüür University of Urbino “Carlo Bo” This important book offers the first coherent gathering of perspectives on the way animals are communicating with each ANIMAL UMWELTEN other and with us as environmental change requires increasing adaptation. Produced by a young generation of zoosemiotics scholars engaged in international research programs at Tartu, IN A CHANGING this work introduces an exciting research field linking the biological sciences with the humanities. Its key premises are that all animals participate in a dynamic web of meanings WORLD: and signs in their own distinctive styles, and all animal spe- cies have distinctive cultures. -

Systematic Review of the Frog Family Hylidae, with Special Reference to Hylinae: Phylogenetic Analysis and Taxonomic Revision
SYSTEMATIC REVIEW OF THE FROG FAMILY HYLIDAE, WITH SPECIAL REFERENCE TO HYLINAE: PHYLOGENETIC ANALYSIS AND TAXONOMIC REVISION JULIAÂ N FAIVOVICH Division of Vertebrate Zoology (Herpetology), American Museum of Natural History Department of Ecology, Evolution, and Environmental Biology (E3B) Columbia University, New York, NY ([email protected]) CEÂ LIO F.B. HADDAD Departamento de Zoologia, Instituto de BiocieÃncias, Unversidade Estadual Paulista, C.P. 199 13506-900 Rio Claro, SaÄo Paulo, Brazil ([email protected]) PAULO C.A. GARCIA Universidade de Mogi das Cruzes, AÂ rea de CieÃncias da SauÂde Curso de Biologia, Rua CaÃndido Xavier de Almeida e Souza 200 08780-911 Mogi das Cruzes, SaÄo Paulo, Brazil and Museu de Zoologia, Universidade de SaÄo Paulo, SaÄo Paulo, Brazil ([email protected]) DARREL R. FROST Division of Vertebrate Zoology (Herpetology), American Museum of Natural History ([email protected]) JONATHAN A. CAMPBELL Department of Biology, The University of Texas at Arlington Arlington, Texas 76019 ([email protected]) WARD C. WHEELER Division of Invertebrate Zoology, American Museum of Natural History ([email protected]) BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY CENTRAL PARK WEST AT 79TH STREET, NEW YORK, NY 10024 Number 294, 240 pp., 16 ®gures, 2 tables, 5 appendices Issued June 24, 2005 Copyright q American Museum of Natural History 2005 ISSN 0003-0090 CONTENTS Abstract ....................................................................... 6 Resumo ....................................................................... -

Home Range Overlap and Density Interacts to Affect Conspecific Encounter Rates in a Gregarious Ungulate
Author's personal copy Behav Ecol Sociobiol DOI 10.1007/s00265-013-1652-0 ORIGINAL PAPER Density dependence in social behaviour: home range overlap and density interacts to affect conspecific encounter rates in a gregarious ungulate Eric Vander Wal & Michel P. Laforge & Philip D. McLoughlin Received: 4 March 2013 /Revised: 5 November 2013 /Accepted: 5 November 2013 # Springer-Verlag Berlin Heidelberg 2013 Abstract Sociality is poorly understood in the context of of these results as they pertain to sociobiology, resource population processes. We used wild, female elk (Cervus competition, and pathogen transmission. canadensis) equipped with proximity-logging radio collars (n =62) from Manitoba, Canada (2007–2009), to test for Keywords Cervus canadensis . Density dependence . Elk . modifying effects of population density (two areas: 0.42 and Encounter rate . Sociality 0.22 animals/km2) on the relationship between two measures of sociality. This included the rate at which collared individ- uals encountered one another per year (encounters logged as Introduction animals ranging to within 1.4 m of each other) and the extent to which animals overlapped in annual home range (propor- Sociality is broadly defined as the properties and processes of tion of shared minimum convex polygon ranges). Overlap social existence (Wilson 1975). Ecologists interested in soci- was significantly greater in the high density area compared ality seek to inform us about the ecological and evolutionary to that of the low, but not if we only considered individuals factors that promote and maintain interactions between con- that directly encountered each other, implying that familiar specifics leading to group living. Despite broad acceptance individuals will maintain a constant degree of range overlap that density-dependent processes are fundamental to ecology, regardless of density.