Omalizumab for Previously Treated Chronic Spon
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BAFF-Neutralizing Interaction of Belimumab Related to Its Therapeutic Efficacy for Treating Systemic Lupus Erythematosus
ARTICLE DOI: 10.1038/s41467-018-03620-2 OPEN BAFF-neutralizing interaction of belimumab related to its therapeutic efficacy for treating systemic lupus erythematosus Woori Shin1, Hyun Tae Lee1, Heejin Lim1, Sang Hyung Lee1, Ji Young Son1, Jee Un Lee1, Ki-Young Yoo1, Seong Eon Ryu2, Jaejun Rhie1, Ju Yeon Lee1 & Yong-Seok Heo1 1234567890():,; BAFF, a member of the TNF superfamily, has been recognized as a good target for auto- immune diseases. Belimumab, an anti-BAFF monoclonal antibody, was approved by the FDA for use in treating systemic lupus erythematosus. However, the molecular basis of BAFF neutralization by belimumab remains unclear. Here our crystal structure of the BAFF–belimumab Fab complex shows the precise epitope and the BAFF-neutralizing mechanism of belimumab, and demonstrates that the therapeutic activity of belimumab involves not only antagonizing the BAFF–receptor interaction, but also disrupting the for- mation of the more active BAFF 60-mer to favor the induction of the less active BAFF trimer through interaction with the flap region of BAFF. In addition, the belimumab HCDR3 loop mimics the DxL(V/L) motif of BAFF receptors, thereby binding to BAFF in a similar manner as endogenous BAFF receptors. Our data thus provides insights for the design of new drugs targeting BAFF for the treatment of autoimmune diseases. 1 Department of Chemistry, Konkuk University, 120 Neungdong-ro, Gwangjin-gu, Seoul 05029, Republic of Korea. 2 Department of Bio Engineering, Hanyang University, 222 Wangsimni-ro, Seongdong-gu, Seoul 04763, Republic of Korea. These authors contributed equally: Woori Shin, Hyun Tae Lee, Heejin Lim, Sang Hyung Lee. -

Xolair/Omalizumab
Therapeutic/Humanized Antibodies ELISA Kits ‘Humanized antibodies” are ‘Animal Antibodies’ that have been modified by recombinant DNA-technology to reduce the overall content of the animal- portion of immunoglobulin so as to increase acceptance by humans or minimize ‘rejection’. By analogy, if the hands of a mouse is actually responsible for grabbing things then ‘humanized-mouse’ will only contain the ‘mouse hands’ and the rest of the body will be human. Since the size of the IgGs is similar in mouse and human, the size of the native mouse IgG and the ‘humanized IgG’ does not significantly change. Antigen recognition property of an antibody actually resides in small portion of the IgG molecule called the ‘antigen binding site or Fab’. Therefore, humanized antibodies contain the minimal portion from the Fab or the epitopes necessary for antigen binding. The process of "humanization of antibodies" is usually applied to animal (mouse) derived monoclonal antibodies for therapeutic use in humans (for example, antibodies developed as anti-cancer drugs). Xolair (Anti-IgE) is an example of humanized IgG. The portion of the mouse IgG that remains in the ‘humanized IgG’ may be recognized as foreign by humans and may result into the generation of “Human Anti-Drug Antibodies (HADA). The presence of anti-Drug antibody (e.g., Human Anti-Rituximab IgG) that may limit the long-term usage the humanized antibody (Rituximab). Not all monoclonal antibodies designed for human therapeutic use need be humanized since many therapies are short-term. The International Nonproprietary Names of humanized antibodies end in “-zumab”, as in “omalizumab”. Humanized antibodies are distinct from chimeric or fusion antibodies. -

Cutaneous Adverse Effects of Biologic Medications
REVIEW CME MOC Selena R. Pasadyn, BA Daniel Knabel, MD Anthony P. Fernandez, MD, PhD Christine B. Warren, MD, MS Cleveland Clinic Lerner College Department of Pathology Co-Medical Director of Continuing Medical Education; Department of Dermatology, Cleveland Clinic; of Medicine of Case Western and Department of Dermatology, W.D. Steck Chair of Clinical Dermatology; Director of Clinical Assistant Professor, Cleveland Clinic Reserve University, Cleveland, OH Cleveland Clinic Medical and Inpatient Dermatology; Departments of Lerner College of Medicine of Case Western Dermatology and Pathology, Cleveland Clinic; Assistant Reserve University, Cleveland, OH Clinical Professor, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, OH Cutaneous adverse effects of biologic medications ABSTRACT iologic therapy encompasses an expo- B nentially expanding arena of medicine. Biologic therapies have become widely used but often As the name implies, biologic therapies are de- cause cutaneous adverse effects. The authors discuss the rived from living organisms and consist largely cutaneous adverse effects of tumor necrosis factor (TNF) of proteins, sugars, and nucleic acids. A clas- alpha inhibitors, epidermal growth factor receptor (EGFR) sic example of an early biologic medication is inhibitors, small-molecule tyrosine kinase inhibitors insulin. These therapies have revolutionized (TKIs), and cell surface-targeted monoclonal antibodies, medicine and offer targeted therapy for an including how to manage these reactions -

(Human) ELISA Kit Rev 06/20 (Catalog # E 4381 - 100, 100 Assays, Store at 4°C) I
FOR RESEARCH USE ONLY! TM BioSim Omalizumab (Human) ELISA Kit rev 06/20 (Catalog # E4381-100, 100 assays, Store at 4°C) I. Introduction: Omalizumab is a recombinant DNA-derived humanized IgG1k monoclonal antibody that selectively binds to human immunoglobulin E (IgE). Omalizumab inhibits the binding of IgE to the high-affinity IgE receptor (FceRI) on the surface of mast cells and basophils. Reduction in surface-bound IgE on FceRI-bearing cells limits the degree of release of mediators of the allergic response. Omalizumab is used to treat severe, persistent asthma. BioSimTM Omalizumab ELISA kit has been developed for specific quantification of Omalizumab concentration in human serum or plasma with high sensitivity and reproducibility. II. Application: This ELISA kit is used for in vitro quantitative determination of Omalizumab Detection Range: 10 - 1000 ng/ml Sensitivity: 10 ng/ml Assay Precision: Intra-Assay: CV < 15%; Inter-Assay: CV < 15% (CV (%) = SD/mean X 100) Recovery rate: 85 – 115% with normal human serum samples with known concentrations Cross Reactivity: There is no cross reaction with native serum immunoglobulins and tested monoclonal antibodies such as infliximab (Remicade®), adalimumab (Humira®), etanercept (Enbrel®), bevacizumab, trastuzumab III. Sample Type: Human serum and plasma IV. Kit Contents: Components E4381-100 Part No. Micro ELISA Plate 1 plate E4381-100-1 Omalizumab Standards (S1 – S7) 1 ml X 7 E4381-100-2.x Assay Buffer 50 ml E4381-100-3 HRP-conjugate Probe 12 ml E4381-100-4 TMB substrate (Avoid light) 12 ml E4381-100-5 Stop Solution 12 ml E4381-100-6 Wash buffer (20X) 50 ml E4381-100-7 Plate sealers 2 E4381-100-8 V. -

(Human) ELISA Kit Rev 07/18 (Catalog # E 4398 - 100, 100 Assays, Store at 4°C) I
FOR RESEARCH USE ONLY! TM BioSim anti-Ipilimumab (Yervoy®) (Human) ELISA Kit rev 07/18 (Catalog # E4398-100, 100 assays, Store at 4°C) I. Introduction: Ipilimumab (Yervoy®) is a fully human IgG1κ antibody that binds to CTLA-4 (cytotoxic T lymphocyteassociated antigen 4), a molecule on T- cells that is indicated for unresectable or metastatic melanoma. The absence or presence of CTLA-4 can augment or suppress the immune system's T-cell response in fighting disease. Ipilimumab is designed to block the activity of CTLA-4, thereby sustaining an active immune response in its attack on cancer cells. The proposed mechanism of action is indirect, and may be through T-cell - mediated anti-tumor immune responses. However, some patients develop unwanted immunogenicity, which leads to production of anti-drug-antibodies (ADAs) inactivating the therapeutic effects of the treatment and, in rare cases, inducing adverse effects. BioVision’s BioSimTM anti-Ipilimumab ELISA kit is designed to detect antibody against Ipilimumab with high specificity and sensitivity in biological matrices. II. Application: This ELISA kit is used for in vitro qualitative determination of antibody against Ipilimumab in serum and plasma Cross Reactivity: Ipilimumab (Yervoy®) infusion camouflages/masks the presence of antibody to Ipilimumab (ATP) in serum/plasma samples. Therefore, blood sampling time is critical for detection of ATP. It is convenient to obtain blood sample just before the infusion or at least 2 weeks after the infusion of Ipilimumab. III. Sample Type: Human serum and plasma IV. Kit Contents: Components E4398-100 Part No. Micro ELISA Plate 1 plate E4398-100-1 Positive Control 0.25 ml E4398-100-2 Negative Control 0.5 ml E4398-100-3 Assay Buffer 12 ml E4398-100-4 Peroxidase Conjugate 12 ml E4398-100-5 TMB substrate (Avoid light) 12 ml E4398-100-6 Stop Solution 12 ml E4398-100-7 Wash buffer (20X) 50 ml E4398-100-8 Plate sealers 2 E4398-100-9 V. -
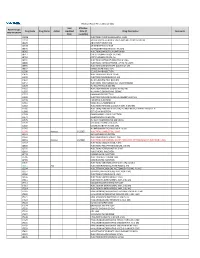
2021 Prior Authorization List Part B Appendix a (PDF)
Medicare Part B PA List Effective 2021 Last Effective Part B Drugs: Drug Code Drug Name Action Updated Date (if Drug Description Comments STEP THERAPY Date available) C9050 INJECTION, EMAPALUMAB-LZSG, 1 MG C9122 MOMETASONE FUROATE SINUS IMPLANT 10 MCG SINUVA J0129 ABATACEPT INJECTION J0178 AFLIBERCEPT INJECTION J0570 BUPRENORPHINE IMPLANT 74.2MG J0585 INJECTION,ONABOTULINUMTOXINA J0717 CERTOLIZUMAB PEGOL INJ 1MG J0718 CERTOLIZUMAB PEGOL INJ J0791 INJECTION CRIZANLIZUMAB-TMCA 5 MG J0800 INJECTION, CORTICOTROPIN, UP TO 40 UNITS J0896 INJECTION LUSPATERCEPT-AAMT 0.25 MG J0897 DENOSUMAB INJECTION J1300 ECULIZUMAB INJECTION J1428 INJECTION ETEPLIRSEN 10 MG J1429 INJECTION GOLODIRSEN 10 MG J1442 INJ FILGRASTIM EXCL BIOSIMIL J1447 INJECTION, TBO-FILGRASTIM, 1 MICROGRAM J1459 INJ IVIG PRIVIGEN 500 MG J1555 INJECTION IMMUNE GLOBULIN 100 MG J1556 INJ, IMM GLOB BIVIGAM, 500MG J1557 GAMMAPLEX INJECTION J1558 INJECTION IMMUNE GLOBULIN XEMBIFY 100 MG J1559 HIZENTRA INJECTION J1561 GAMUNEX-C/GAMMAKED J1562 INJECTION; IMMUNE GLOBULIN 10%, 5 GRAMS J1566 INJECTION, IMMUNE GLOBULIN, INTRAVENOUS, LYOPHILIZED (E.G. P J1568 OCTAGAM INJECTION J1569 GAMMAGARD LIQUID INJECTION J1572 FLEBOGAMMA INJECTION J1575 INJ IG/HYALURONIDASE 100 MG IG J1599 IVIG NON-LYOPHILIZED, NOS J1602 GOLIMUMAB FOR IV USE 1MG J1745 INJ INFLIXIMAB EXCL BIOSIMILR 10 MG J1930 Remove 1/1/2021 INJECTION, LANREOTIDE, 1 MG J2323 NATALIZUMAB INJECTION J2350 INJECTION OCRELIZUMAB 1 MG J2353 Remove 1/1/2021 INJECTION, OCTREOTIDE, DEPOT FORM FOR INTRAMUSCULAR INJECTION, 1 MG J2357 INJECTION, OMALIZUMAB, -

Cimzia (Certolizumab Pegol) AHM
Cimzia (Certolizumab Pegol) AHM Clinical Indications • Cimzia (Certolizumab Pegol) is considered medically necessary for adult members 18 years of age or older with moderately-to-severely active disease when ALL of the following conditions are met o Moderately-to-severely active Crohn's disease as manifested by 1 or more of the following . Diarrhea . Abdominal pain . Bleeding . Weight loss . Perianal disease . Internal fistulae . Intestinal obstruction . Megacolon . Extra-intestinal manifestations: arthritis or spondylitis o Crohn's disease has remained active despite treatment with 1 or more of the following . Corticosteroids . 6-mercaptopurine/azathioprine • Certollizumab pegol (see note) is considered medically necessary for persons with active psoriatic arthritis who meet criteria in Psoriasis and Psoriatic Arthritis: Biological Therapies. • Cimzia, (Certolizumab Pegol), alone or in combination with methotrexate (MTX), is considered medically necessary for the treatment of adult members 18 years of age or older with moderately-to-severely active rheumatoid arthritis (RA). • Cimzia (Certolizumab pegol is considered medically necessary for reducing signs and symptoms of members with active ankylosing spondylitis who have an inadequate response to 2 or more NSAIDs. • Cimzia (Certolizumab Pegol) is considered investigational for all other indications (e.g.,ocular inflammation/uveitis; not an all-inclusive list) because its effectiveness for indications other than the ones listed above has not been established. Notes • There are several brands of targeted immune modulators on the market. There is a lack of reliable evidence that any one brand of targeted immune modulator is superior to other brands for medically necessary indications. Enbrel (etanercept), Humira (adalimumab), Remicade (infliximab), Simponi (golimumab), Simponi Aria (golimumab intravneous), and Stelara (ustekinumab) brands of targeted immune modulators ("least cost brands of targeted immune modulators") are less costly to the plan. -

Anti-TNF-Α (Certolizumab), Humanized Antibody
FOR RESEARCH USE ONLY! rev 06/21 Anti-TNF-α (Certolizumab), Humanized Antibody CATALOG NO.: A2142-100 (100 µg) BACKGROUND DESCRIPTION: The research-grade biosimilar is a humanized TNF-α monoclonal antibody. The original monoclonal antibody (Certolizumab pegol (CZP)) consists of polyethylene glycol conjugated to the antigen-binding fragment of the humanized monoclonal antibody. The antibody targets and neutralizes soluble and membrane forms of tumor necrosis factor-alpha (TNF-α) and inhibits TNF-α mediated signaling in vitro. Unlike other TNF inhibitors, this antibody does not contain the Fc region. As a result, decreased Fc-mediated effects such as antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) are observed. The original monoclonal antibody received FDA approval to treat moderate to severe rheumatoid arthritis, Crohn’s disease, ankylosing spondylitis, and psoriatic arthritis. TNFSF2, TNF-alpha, TNFA, TNF-α, TNFα ALTERNATE NAMES: ANTIBODY TYPE: Monoclonal HOST/ISOTYPE: Recombinant / Fab-IgG1-kappa SOURCE: CHO cells IMMUNOGEN: Human TNF-α FORM: Liquid FORMULATION: In PBS, pH 7.5 SPECIES REACTIVITY: Human STORAGE CONDITIONS: Store at -80 ºC. Avoid repeated freeze-thaw cycles This information is only intended as a guide. The optimal dilutions must be determined by the user RELATED PRODUCTS: Anti-TNF-α (Adalimumab), humanized Antibody (Cat. No. A1048) Anti-TNF alpha (Infliximab), Human IgG1 Antibody (Cat. No. A1097) Anti-TNF alpha (Humicade), Human IgG4 Antibody (Cat. No. A1093) Anti-EGFR (Cetuximab), Chimeric Antibody (Cat. No. A1047) Anti-EGFR (Matuzumab), Human IgG1 Antibody (Cat. No. A1090) FOR RESEARCH USE ONLY! Not to be used on humans. 155 S. Milpitas Blvd., Milpitas, CA 95035 USA | T: (408)493-1800 F: (408)493-1801 | www.biovision.com | [email protected] . -

SUPPLEMENTARY APPENDIX 4: Search Strategies Syntax Guide For
SUPPLEMENTARY APPENDIX 4: Search Strategies Pubmed, Embase Perioperative Management - PubMed Search Strategy – March 6, 2016 Syntax Guide for PubMed [MH] = Medical Subject Heading, also [TW] = Includes all words and numbers in known as MeSH the title, abstract, other abstract, MeSH terms, MeSH Subheadings, Publication Types, Substance Names, Personal Name as Subject, Corporate Author, Secondary Source, Comment/Correction Notes, and Other Terms - typically non-MeSH subject terms (keywords)…assigned by an organization other than NLM [SH] = a Medical Subject Heading [TIAB] = Includes words in the title and subheading, e.g. drug therapy abstracts [MH:NOEXP] = a command to retrieve the results of the Medical Subject Heading specified, but not narrower Medical Subject Heading terms Boolean Operators OR = retrieves results that include at least AND = retrieves results that include all the one of the search terms search terms NOT = excludes the retrieval of terms from the search Perioperative Management PubMed Search Strategy – March 6, 2016 Search Query #1 ((((ARTHROPLASTY, REPLACEMENT, HIP[MH] OR HIP PROSTHES*[TW] OR HIP REPLACEMENT*[TIAB] OR HIP ARTHROPLAST*[TIAB] OR HIP TOTAL REPLACEMENT*[TIAB] OR FEMORAL HEAD PROSTHES*[TIAB]) OR (ARTHROPLASTY, REPLACEMENT, KNEE[MH] OR KNEE PROSTHES*[TW] OR KNEE REPLACEMENT*[TW] OR KNEE ARTHROPLAST*[TW] OR KNEE TOTAL REPLACEMENT*[TIAB]) OR (ARTHROPLAST*[TW] AND (HIP[TIAB] OR HIPS[TIAB] OR KNEE*[TIAB])) AND (("1980/01/01"[PDAT] : "2016/03/06"[PDAT]) AND ENGLISH[LANG])) NOT (((("ADOLESCENT"[MESH]) OR "CHILD"[MESH]) -

(12) Patent Application Publication (10) Pub. No.: US 2017/0172932 A1 Peyman (43) Pub
US 20170172932A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0172932 A1 Peyman (43) Pub. Date: Jun. 22, 2017 (54) EARLY CANCER DETECTION AND A 6LX 39/395 (2006.01) ENHANCED IMMUNOTHERAPY A61R 4I/00 (2006.01) (52) U.S. Cl. (71) Applicant: Gholam A. Peyman, Sun City, AZ CPC .......... A61K 9/50 (2013.01); A61K 39/39558 (US) (2013.01); A61K 4I/0052 (2013.01); A61 K 48/00 (2013.01); A61K 35/17 (2013.01); A61 K (72) Inventor: sham A. Peyman, Sun City, AZ 35/15 (2013.01); A61K 2035/124 (2013.01) (21) Appl. No.: 15/143,981 (57) ABSTRACT (22) Filed: May 2, 2016 A method of therapy for a tumor or other pathology by administering a combination of thermotherapy and immu Related U.S. Application Data notherapy optionally combined with gene delivery. The combination therapy beneficially treats the tumor and pre (63) Continuation-in-part of application No. 14/976,321, vents tumor recurrence, either locally or at a different site, by filed on Dec. 21, 2015. boosting the patient’s immune response both at the time or original therapy and/or for later therapy. With respect to Publication Classification gene delivery, the inventive method may be used in cancer (51) Int. Cl. therapy, but is not limited to such use; it will be appreciated A 6LX 9/50 (2006.01) that the inventive method may be used for gene delivery in A6 IK 35/5 (2006.01) general. The controlled and precise application of thermal A6 IK 4.8/00 (2006.01) energy enhances gene transfer to any cell, whether the cell A 6LX 35/7 (2006.01) is a neoplastic cell, a pre-neoplastic cell, or a normal cell. -

Omalizumab, Xolair
XOLAIR® Omalizumab For Subcutaneous Use DESCRIPTION Xolair (Omalizumab) is a recombinant DNA-derived humanized IgG1k monoclonal antibody that selectively binds to human immunoglobulin E (IgE). The antibody has a molecular weight of approximately 149 kilodaltons. Xolair is produced by a Chinese hamster ovary cell suspension culture in a nutrient medium containing the antibiotic gentamicin. Gentamicin is not detectable in the final product. Xolair is a sterile, white, preservative-free, lyophilized powder contained in a single-use vial that is reconstituted with Sterile Water for Injection (SWFI), USP, and administered as a subcutaneous (SC) injection. A Xolair vial contains 202.5 mg of Omalizumab, 145.5 mg sucrose, 2.8 mg L-histidine hydrochloride monohydrate, 1.8 mg L-histidine and 0.5 mg polysorbate 20 and is designed to deliver 150 mg of Omalizumab, in 1.2 mL after reconstitution with 1.4 mL SWFI, USP. CLINICAL PHARMACOLOGY Mechanism of Action Xolair inhibits the binding of IgE to the high-affinity IgE receptor (FceRI) on the surface of mast cells and basophils. Reduction in surface bound IgE on FceRI-bearing cells limits the degree of release of mediators of the allergic response. Treatment with Xolair also reduces the number of FceRI receptors on basophils in atopic patients. Pharmacokinetics After SC administration, Omalizumab is absorbed with an average absolute bioavailability of 62%. Following a single SC dose in adult and adolescent patients with asthma, Omalizumab was absorbed slowly, reaching peak serum concentrations after an average of 7-8 days. The pharmacokinetics of Omalizumab are linear at doses greater than 0.5 mg/kg. -

Immunopharmacology: a Guide to Novel Therapeutic Tools - Francesco Roselli, Emilio Jirillo
PHARMACOLOGY – Vol. II - Immunopharmacology: A Guide to Novel Therapeutic Tools - Francesco Roselli, Emilio Jirillo IMMUNOPHARMACOLOGY: A GUIDE TO NOVEL THERAPEUTIC TOOLS Francesco Roselli Department of Neurological and Psychiatric Sciences, University of Bari, Bari, Italy Emilio Jirillo Department of Internal Medicine, Immunology and Infectious Disease, University of Bari, Italy National Institute for Digestive Disease, Castellana Grotte, Bari, Italy Keywords: Immunopharmacology, immunosuppressive agents, immunomodulating agents, Rituximab, Natalizumab, Efalizumab, Abatacept, Betalacept, Alefacept, Basiliximab, Daclizumab, Infliximab, Etanercept, Adalimumab, Anakinra, Tocilizumab, Omalizumab, Interleukin-2, Denileukin diftitox, Interferon-γ, Interleukin-12 Contents 1. Introduction 2. B cell targeted molecule: Rituximab 3. Lymphocyte trafficking inhibitors: Natalizumab and Efalizumab 3.1 Natalizumab 3.2 Efalizumab 4. Costimulation antagonists: Abatacept, Betalacept, Alefacept 4.1 Abatacept 4.2 Betalacept 4.3 Alefacept 5. Interleukin-2 Receptor antagonists: Basiliximab, Daclizumab 5.1 Basiliximab 5.2 Daclizumab 6. Antagonists of soluble mediators of inflammation 6.1 TNF-α antagonists: Infliximab, Etanercept, Adalimumab 6.1.1 Infliximab 6.1.2 Etanercept 6.1.3 Adalimumab 6.2 Interleukin-1UNESCO Receptor Antagonist (Anakinra) – EOLSS 6.3 Interleukin-6 receptor antagonist (tocilizumab) 7. Antagonist of IgE: Omalizumab 8. Interleukin therapySAMPLE in oncology CHAPTERS 8.1 Interleukin-2 8.2 Interleukin-2/diphtheria toxin conjugate (Ontak) 8.3 Interferon-γ and Interleukin-12 9. Perspectives and future developments Glossary Bibliography Biographical Sketches Summary ©Encyclopedia of Life Support Systems (EOLSS) PHARMACOLOGY – Vol. II - Immunopharmacology: A Guide to Novel Therapeutic Tools - Francesco Roselli, Emilio Jirillo Immunopharmacology is that area of pharmacological sciences dealing with the selective modulation (i.e. upregulation or downregulation) of specific immune responses and, in particular, of immune cell subsets.