Physiological Bases for the Temperature-Dependent Short Growth of Hypocotyls in Some Varieties of Soybean Cyrus Samimy Iowa State University
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evaluating Ethylene Sensitivity Using Mature Plant Screens and the Seedling Hypocotyl Response
Evaluating Ethylene Sensitivity Using Mature Plant Screens and the Seedling Hypocotyl Response THESIS Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Nichole Frances Edelman, B. A. Graduate Program in Horticulture and Crop Science The Ohio State University 2013 Master's Examination Committee: Dr. Michelle L. Jones, Advisor Dr. Pablo Jourdan Dr. Claudio Pasian Copyrighted by Nichole Frances Edelman 2013 Abstract Ethylene (C2H4) is a gaseous hormone produced by plants in response to environmental stress and during growth and development. Sensitive seedlings exposed to ethylene gas will exhibit an exaggerated apical hook, thickened hypocotyl, and reduced hypocotyl elongation. Collectively these symptoms are known as the triple response, and have been used as a screen to identify ethylene mutants. Exposure to ethylene gas at the mature plant stage can induce flower, bud, or leaf abscission, flower senescence, leaf chlorosis (yellowing), or leaf epinasty (downward curvature). Sensitivity can vary between species and by developmental stage or tissue (flower vs leaf). Ethylene can damage plants during production, shipping, and retailing. The objectives of this research were 1) to determine if the seedling hypocotyl elongation screen could be used to predict the sensitivity of mature plants at the marketable stage; 2) to identify ethylene sensitivity differences (levels of sensitivity and symptoms) between accessions within Solanaceae; and 3) to identify ethylene sensitivity differences at different developmental stages (seedling, juvenile, and mature plants). Seedlings were germinated on filter paper saturated with 1- aminocyclopropane-1-carboxylic acid (ACC) and sensitivity was determined based on the hypocotyl lengths relative to the control (0 µM ACC). -
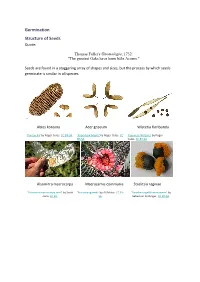
Germination Structure of Seeds Quote
Germination Structure of Seeds Quote: Thomas Fuller's Gnomologia, 1732: "The greatest Oaks have been little Acorns." Seeds are found in a staggering array of shapes and sizes, but the process by which seeds germinate is similar in all species. Abies koreana Acer griseum Wisteria floribunda 'Korean Fir' by Roger Culos. CC BY-SA. 'Paperbark Maple' by Roger Culos. CC 'Japanese Wisteria' by Roger BY-SA. Culos. CC BY-SA. Alsomitra macrocarpa Macrozamia communis Strelitzia reginae 'Alsomitra macrocarpa seed' by Scott 'BurrawangSeeds' by AYArktos. CC BY- 'Paradiesvogelblumensamen' by Zona. CC BY. SA. Sebastian Stabinger. CC BY-SA. Taraxicum officinale Stephanotis floribunda Phleum pratense 'Achane of Taraxacum sect. 'Stephanotis seed' by L. Marie"/Lenore 'Timoteegras vruchten Phleum Ruderalia' by Didier Edman, Sunnyvale, CA. CC BY. pratense' by Rasbak. CC BY-SA. Descouens. CC BY-SA. Dicotyledon seeds testa epicotyl plumule hypocotyl cotyledon radicle 'Aesculus hippocastanum seed section' by Boronian. CC BY. plumule epicotyl hypocotyl testa hilum radicle cotyledon micropyle endosperm Monocotyledon seeds endosperm epicotyl testa hypocotyl cotyledon radicle Parts of a seed Testa The seed coat. A protective layer which is tough and hard and it protects the seed from attack by insects, fungi and bacteria. Cotyledon Dicotyledons have 2 cotyledons Monocotyledons have 1 cotyledon A cotyledon is an embryonic leaf. It is the first leaf to appear when a seedling grows. They often contain reserves of food which the developing seedling can use to grow. Epicotyl The section of stem between the cotyledon(s) and the plumule. In a seedling it is the section of stem between the cotyledons and the first true leaves. -
4-H Growing Things Series Discovering the Science of Plants Welcome 4-H Leaders!
4-H Growing Things Series Discovering the Science of Plants Welcome 4-H Leaders! Welcome to the “Discovering the Science of Plants” project. There is lots of information, fun facts and hands on activities that cover the basic scientific Table of Contents principles of how plants grow and survive. This guide provides you with Introduction 1 project meeting plans (Skill Builders) that include, a skills list, background information, activity suggestions, and ways to know if your members have Project Summary 2 learned the skills identified. Skill Builder 1: 5 What is a plant? In this project, members will examine, by learning to do by doing, how plants grow and how they are used in everyday life. The Leader Guide is written with Skill Builder 2: 11 the expectation that the project leader(s) will have a working knowledge about Seeds for the the project topics and how they work. If not, you may need to do some Future pre-work / research on the activities, or recruit assistance for certain sections. Skill Builder 3: 18 Getting to the Be sure to try out activities, demonstrations or hands on work ahead of time to Root of it ensure you have an understanding of each Skill Builder - this also allows for any adjustments should an activity not work for you or if any equipment or supplies Skill Builder 4: 26 are unavailable. Leaves and Stems: the Power The 3D’s of Learning - Each Skill Builder has three sections of learning called Generators “Dream it!”, “Do it!” and “Dig it!”. Below is a description of each. -

Drupe. Fruit with a Hard Endocarp (Figs. 67 and 71-73); E.G., and Sterculiaceae (Helicteres Guazumaefolia, Sterculia)
Fig. 71. Fig. 72. Fig. 73. Drupe. Fruit with a hard endocarp (figs. 67 and 71-73); e.g., and Sterculiaceae (Helicteres guazumaefolia, Sterculia). Anacardiaceae (Spondias purpurea, S. mombin, Mangifera indi- Desmopsis bibracteata (Annonaceae) has aggregate follicles ca, Tapirira), Caryocaraceae (Caryocar costaricense), Chrysobal- with constrictions between successive seeds, similar to those anaceae (Licania), Euphorbiaceae (Hyeronima), Malpighiaceae found in loments. (Byrsonima crispa), Olacaceae (Minquartia guianensis), Sapin- daceae (Meliccocus bijugatus), and Verbenaceae (Vitex cooperi). Samaracetum. Aggregate of samaras (fig. 74); e.g., Aceraceae (Acer pseudoplatanus), Magnoliaceae (Liriodendron tulipifera Hesperidium. Septicidal berry with a thick pericarp (fig. 67). L.), Sapindaceae (Thouinidium dodecandrum), and Tiliaceae Most of the fruit is derived from glandular trichomes. It is (Goethalsia meiantha). typical of the Rutaceae (Citrus). Multiple Fruits Aggregate Fruits Multiple fruits are found along a single axis and are usually coalescent. The most common types follow: Several types of aggregate fruits exist (fig. 74): Bibacca. Double fused berry; e.g., Lonicera. Achenacetum. Cluster of achenia; e.g., the strawberry (Fra- garia vesca). Sorosis. Fruits usually coalescent on a central axis; they derive from the ovaries of several flowers; e.g., Moraceae (Artocarpus Baccacetum or etaerio. Aggregate of berries; e.g., Annonaceae altilis). (Asimina triloba, Cananga odorata, Uvaria). The berries can be aggregate and syncarpic as in Annona reticulata, A. muricata, Syconium. Syncarp with many achenia in the inner wall of a A. pittieri and other species. hollow receptacle (fig. 74); e.g., Ficus. Drupacetum. Aggregate of druplets; e.g., Bursera simaruba THE GYMNOSPERM FRUIT (Burseraceae). Fertilization stimulates the growth of young gynostrobiles Folliacetum. Aggregate of follicles; e.g., Annonaceae which in species such as Pinus are more than 1 year old. -

Osbhlh073 Negatively Regulates Internode Elongation and Plant Height by Modulating GA Homeostasis in Rice
plants Article OsbHLH073 Negatively Regulates Internode Elongation and Plant Height by Modulating GA Homeostasis in Rice 1, 2, 3 4 2 Jinwon Lee y, Sunok Moon y, Seonghoe Jang , Sichul Lee , Gynheung An , Ki-Hong Jung 2,* and Soon Ki Park 1,* 1 School of Applied Biosciences, Kyungpook National University, Daegu 41566, Korea; [email protected] 2 Graduate School of Biotechnology & Crop Biotech Institute, Kyung Hee University, Yongin 17104, Korea; [email protected] (S.M.); [email protected] (G.A.) 3 World Vegetable Center Korea Office (W.K.O), Jellabuk-do 55365, Korea; [email protected] 4 Center for Plant Aging Research, Institute for Basic Science (IBS), Daegu 42988, Korea; [email protected] * Correspondence: [email protected] (K.-H.J.); [email protected] (S.K.P.) These authors contributed equally to this work: J.L., S.M. y Received: 5 March 2020; Accepted: 21 April 2020; Published: 23 April 2020 Abstract: Internode elongation is one of the key agronomic traits determining a plant’s height and biomass. However, our understanding of the molecular mechanisms controlling internode elongation is still limited in crop plant species. Here, we report the functional identification of an atypical basic helix-loop-helix transcription factor (OsbHLH073) through gain-of-function studies using overexpression (OsbHLH073-OX) and activation tagging (osbhlh073-D) lines of rice. The expression of OsbHLH073 was significantly increased in the osbhlh073-D line. The phenotype of osbhlh073-D showed semi-dwarfism due to deficient elongation of the first internode and poor panicle exsertion. Transgenic lines overexpressing OsbHLH073 confirmed the phenotype of the osbhlh073-D line. -

Tulipa Kaufmanniana Regel.) Propagation Under Ex Situ Conditions ISSN 2447-536X |
THE APPLICATION OF DIFFERENT REPRODUCTION TECHNIQUES FOR RARE SPECIES WATERLILY TULIP 450 Ornamental (HorticultureTULIPA KAUFMANNIANA REGEL.) PROPAGATION UNDER EX SITU CONDITIONS ISSN 2447-536X | HTTPS://ORNAMENTALHORTICULTURE.EMNUVENS.COM.BR/RBHO SCIENTIFIC ARTICLE The application of different reproduction techniques for rare species waterlily tulip (Tulipa kaufmanniana Regel.) propagation under ex situ conditions Nabieva Alexandra Yurievna1*, Gerasimovich Lyudmila Vladimirovna2 Abstract T. kaufmanniana is a threatened and endemic species of the Tien Shan Mountains with a complex of valuable ornamental features. However, the commercial usage of the plant is limited due to the restricted availability of the bulbs in nature, difficulties in overcoming seed dormancy and low efficiency of the species reproduction. Results of the investigation of the reproductive biology of this species in the culture conditions allowed us to characterize T. kaufmanniana as viable with successful seedage. In the work reported here the effect of low temperature on the proper development of embryos is addressed, and an attempt is made to clarify the effect of cytokinins (TDZ, BAP) and auxin (NAA) on the regeneration capacity of isolated T. kaufmanniana embryos for the adventitious shoots and bulblets formation. Direct shoot organogenesis was induced by the combined action of chilling and BAP or TDZ treatment. Among the variants tested, TDZ promoted the formation of adventitious bulbs in 48 % of the chilled embryos, compared to non-chilled embryos (0.0%) and embryos exposed to the same concentration of BAP (39%). The best culture condition for T. kaufmanniana embryos growth and bulblets formation consisted of chilling for 10 wk in the dark at 4 °C, 4 wk at 20 °C under 16 h light photoperiod and 10 wk at 4 °C in the dark. -

Early Soybean Growth and Development
EARLY SOYBEAN GROWTH AND DEVELOPMENT A soybean seed has two distinct parts: the cotyledons and the embryo. The two cotyledons are the main food storage structure, which supply food during emergence and for the seven to ten days after emergence through the V1 growth stage. The embryo itself is comprised of three parts. 1. The radicle is the first root and it’s the first part of the embryo to penetrate the seed coat. Lateral roots form from the radicle, and tiny root hairs then develop on the lateral roots. Root hairs are the main absorbing surface of the entire root system. A soybean’s root system branches and rebranches within the first four to five weeks after planting, with the bulk of root mass persisting in the top six inches of soil. 2. The hypocotyl is the stem below the cotyledon. It begins to elongate after the radicle and forms an arch, which is pushed upward. As the arch breaks the soil surface, it pulls the cotyledons and epicotyl up. The uppermost cells of the hypocotyl stop growing as cells on the underside continue to straighten the arch. This process lifts the cotyledons into an upright position. 3. The epicotyl is comprised of the small leaves, stem and the apical growing point. The epicotyl is protected between the cotyledons until after emergence. The loss of a cotyledon is not lethal to the seedling, but damage to the epicotyl is. The hypocotyl arch is a large structure that is relative to seed size, utilizing considerable seed energy to push through the soil. -

The Effect of Light Intensity on the Hypocotyl Length of Arabidopsis Thaliana Christina Bell, Mandy Choi, Audrey Lam, and Kimber
The effect of light intensity on the hypocotyl length of Arabidopsis thaliana Christina Bell, Mandy Choi, Audrey Lam, and Kimberley Xiao Abstract The amount of light a plant receives during germination can greatly affect its growth and morphology. In this study, seeds of Arabidopsis thaliana were used to investigate the effects of light intensity on hypocotyl growth and length. Seeds of both the wild type and the mutant cer10 of Arabidopsis thaliana were grown in petri dishes for a period of eleven days, under three varying light intensities. These were marked no light, low light and high light, averaging at 3 lux, 141 lux, and 6969 lux, respectively. It was found that for both the mutant and the wild type, seeds grown in a no light environment had a significantly longer hypocotyl (embryonic stem) length than those grown at high light intensity. For the wild type, at the end of the 11th day, those grown in the high light treatment had a mean hypocotyl length of 0.7±0.05mm, those grown in the low light treatment had a mean hypocotyl length of 4.0±0.8mm, and those grown in the no light treatment had a mean hypocotyl length of 10.5±1.3mm. For the mutant cer10, the high light treatment had a mean hypocotyl length of 1.0±0.2mm, the low light treatment had a mean hypocotyl length of 4.0±0.8mm, and the no light treatment had a mean hypocotyl length of 11.8±0.6mm. The results agree with current literature on the subject; however we fail to reject our initial null hypothesis for both the mutant and wild type. -

Agricultural Marketing Service, USDA § 201.56–10
Agricultural Marketing Service, USDA § 201.56–10 (A) One or more essential structures and become thin, leaf-like, and photo- impaired as a result of decay from pri- synthetic. mary infection. (3) Shoot system: The hypocotyl (B) Albino. elongates carrying the cotyledons above the soil surface. The epicotyl [59 FR 64504, Dec. 14, 1994] usually does not show any development § 201.56–8 Flax family, Linaceae. within the test period. Areas of yel- lowish pigmentation may develop on Kind of seed: Flax. the hypocotyl in cotton. (a) General description. (4) Root system: A primary root, with (1) Germination habit: Epigeal dicot. secondary roots usually developing (Due to the mucilaginous nature of the within the test period. Areas of yel- seed coat, seedlings germinated on lowish pigmentation may develop on blotters may adhere to the blotter and the root in cotton. appear to be negatively geotropic.) (b) Abnormal seedling description. (2) Food reserves: Cotyledons which (1) Cotyledons: expand and become photosynthetic. (i) Less than half of the original cot- (3) Shoot system: The hypocotyl yledon tissue remaining attached. elongates carrying the cotyledons (ii) Less than half of the original cot- above the soil surface. The epicotyl yledon tissue free of necrosis or decay. usually does not show any development (Remove any attached seed coats at within the test period. the end of the test period for evalua- (4) Root system: A primary root, with tion of cotyledons.) secondary roots usually developing (2) Epicotyl: within the test period. (i) Missing. (May be assumed to be (b) Abnormal seedling description. present if both cotyledons are intact.) (1) Cotyledons: (ii) [Reserved] (i) Less than half of the original cot- (3) Hypocotyl: yledon tissue remaining attached. -

Federal Aviation Agency
FEDERAL REGISTER VOLUME 30 • NUMBER 117 Friday, June 18,1965 • Washington, D.C. Pages 7863-7938 Agencies in this issue— Agricultural Research Service Area Redevelopment Administration Atomic Energy Commission Civil Aeronautics Board Civil Service Commission Consumer and Marketing Service Federal Aviation Agency Federal Communications Commission Federal Maritime Commission Federal Trade Commission Food and Drug Administration Interstate Commerce Commission Land Management Bureau Securities and Exchange Commission Detailed list of Contents appears inside. Subscriptions Now Being Accepted S L I P L A W S 89th Congress, 1st Session 1965 Separate prints of Public Laws, published immediately after enactment, with marginal annotations and legislative history references. Subscription Price: $12.00 per Session Published by Office of the Federal Register, National Archives and Records Service, General Services Administration Order from Superintendent of Documents, U.S. Government Printing Office, Washington, D.C.,' 20402 Published dally, Tuesday through Saturday (no publication on Sundays, Monday», or FEDERAL®REGISTER on the day after an official Federal holiday), by the Office of the Federal Register, National Area Code 202 Phone 963-3261 A1®1117®8 a n d R ecords Service, G eneral Services A d m in istra tio n (m ail address Nations _ . , _ _ Archives Building, Washington, D.C. 20408), p u r s u a n t to the authority contained in tne Federal Register Act, approved July 26, 1935 (49 Stat. 500, as amended; 44 U.S.C., ch. 8B), under regulations prescribed by the Admin istrative Committee of the Federal Register, approved by the President (1 CFR Ch. I). Distribution is made only by the Superintendent or Documents, Government Printing Office, Washington, D.C. -

Soybean Temperature-Dependent Hypocotyl Growth Anomaly: Physiological Aspects Robert Dean Keys Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1979 Soybean temperature-dependent hypocotyl growth anomaly: physiological aspects Robert Dean Keys Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Botany Commons Recommended Citation Keys, Robert Dean, "Soybean temperature-dependent hypocotyl growth anomaly: physiological aspects " (1979). Retrospective Theses and Dissertations. 6651. https://lib.dr.iastate.edu/rtd/6651 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This was produced from a copy of a document sent to us for microfîlming. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided to help you understand markings or notations which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting through an image and duplicating adjacent pages to assure you of complete continuity. 2. When an image on the film is obliterated with a round black mark it is an indication that the film inspector noticed either blurred copy because of movement during exposure, or duplicate copy. -

Journal of the American Horticultural Society, Inc
Journal of the American Horticultural Society, Inc. 901 NORTH WASHINGTON STREET, ALEXANDRIA, VIRGINIA 22314 For United Horticulture ... The particular objec.ts and business of the American Horticultural Society a.ye to promote and encourage national in·terest in scientific research and education in horticulture in all of its branches. 1970-71 EXECUTIVE COMMITTEE (*) President Treasurer DR. DAVID G. LEACH (1971) MR. JOHN M. PATEK (1972) 1674 Trinity Road President North Madison, Ohio 44057 Color Data, Inc. 434 Mount Airy Drive First Vice President Rochester, New York 14617 MRS. ERNESTA D. BALLARD (1973) Director, Pennsylvania MembeT of the Board Horticultural Society DR. HENRY M. CATHEY (1973) 325 Walnut Street Leader, Ornamental Investigations Philadelphia, Pennsylvania 19106 Agricultural Research Service Second V ice President U.S. Department of Agriculture Beltsville, \!faryland 20705 MR. FREDERICK J. CLOSE (1971) 6189 Shore Drive Immediate Past President North Madison, Ohio 44057 MR. FRED C. GALLE (1971) (U) Secretary Director of Horticulture DR. GEORGE H. M. LAWRENCE (1971) Callaway Gardens 390 Forge Road Pine Mountain, Georgia 31822 East Greenwich, Rhode Island 02818 MR. O. KEISTER EVANS Executive Director Washington, D. C. (e) Members of the 1970-71 Board of Directors per bylaw provision. ( ..) Ex officio, and without vote. THE AMERICAN HORTICULTURAL MAGAZINE is the official publication of The American Horticultural Society and is issued during the Winter, Spring, Summer, and Fall quarters. The magazine is included as a benefit of membership in The American Horticultural Society, individual membership dues being $15 .00 a year. THE AMERICAN HORTICULTURAL MAGAZINE is devoted to the dissemination of knowledge in the science and art of growing ornamental plants, fruits, vegetables, and related subjects.