Data Dictionary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Properties and Units in Clinical Pharmacology and Toxicology
Pure Appl. Chem., Vol. 72, No. 3, pp. 479–552, 2000. © 2000 IUPAC INTERNATIONAL FEDERATION OF CLINICAL CHEMISTRY AND LABORATORY MEDICINE SCIENTIFIC DIVISION COMMITTEE ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)# and INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY CHEMISTRY AND HUMAN HEALTH DIVISION CLINICAL CHEMISTRY SECTION COMMISSION ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)§ PROPERTIES AND UNITS IN THE CLINICAL LABORATORY SCIENCES PART XII. PROPERTIES AND UNITS IN CLINICAL PHARMACOLOGY AND TOXICOLOGY (Technical Report) (IFCC–IUPAC 1999) Prepared for publication by HENRIK OLESEN1, DAVID COWAN2, RAFAEL DE LA TORRE3 , IVAN BRUUNSHUUS1, MORTEN ROHDE1, and DESMOND KENNY4 1Office of Laboratory Informatics, Copenhagen University Hospital (Rigshospitalet), Copenhagen, Denmark; 2Drug Control Centre, London University, King’s College, London, UK; 3IMIM, Dr. Aiguader 80, Barcelona, Spain; 4Dept. of Clinical Biochemistry, Our Lady’s Hospital for Sick Children, Crumlin, Dublin 12, Ireland #§The combined Memberships of the Committee and the Commission (C-NPU) during the preparation of this report (1994–1996) were as follows: Chairman: H. Olesen (Denmark, 1989–1995); D. Kenny (Ireland, 1996); Members: X. Fuentes-Arderiu (Spain, 1991–1997); J. G. Hill (Canada, 1987–1997); D. Kenny (Ireland, 1994–1997); H. Olesen (Denmark, 1985–1995); P. L. Storring (UK, 1989–1995); P. Soares de Araujo (Brazil, 1994–1997); R. Dybkær (Denmark, 1996–1997); C. McDonald (USA, 1996–1997). Please forward comments to: H. Olesen, Office of Laboratory Informatics 76-6-1, Copenhagen University Hospital (Rigshospitalet), 9 Blegdamsvej, DK-2100 Copenhagen, Denmark. E-mail: [email protected] Republication or reproduction of this report or its storage and/or dissemination by electronic means is permitted without the need for formal IUPAC permission on condition that an acknowledgment, with full reference to the source, along with use of the copyright symbol ©, the name IUPAC, and the year of publication, are prominently visible. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Partial Agreement in the Social and Public Health Field
COUNCIL OF EUROPE COMMITTEE OF MINISTERS (PARTIAL AGREEMENT IN THE SOCIAL AND PUBLIC HEALTH FIELD) RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies, and superseding Resolution AP (82) 2) AND APPENDIX I Alphabetical list of medicines adopted by the Public Health Committee (Partial Agreement) updated to 1 July 1988 APPENDIX II Pharmaco-therapeutic classification of medicines appearing in the alphabetical list in Appendix I updated to 1 July 1988 RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (superseding Resolution AP (82) 2) (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies) The Representatives on the Committee of Ministers of Belgium, France, the Federal Republic of Germany, Italy, Luxembourg, the Netherlands and the United Kingdom of Great Britain and Northern Ireland, these states being parties to the Partial Agreement in the social and public health field, and the Representatives of Austria, Denmark, Ireland, Spain and Switzerland, states which have participated in the public health activities carried out within the above-mentioned Partial Agreement since 1 October 1974, 2 April 1968, 23 September 1969, 21 April 1988 and 5 May 1964, respectively, Considering that the aim of the Council of Europe is to achieve greater unity between its members and that this -

North Carolina Trauma Registry (NCTR) Data Dictionary NCTR Data Dictionary
North Carolina Trauma Registry (NCTR) Data Dictionary NCTR Data Dictionary Introduction This document, the North Carolina Trauma Registry (NCTR) Data Dictionary, was created using the data dictionary published by the National Trauma Registry of the American College of Surgeons (NTRACS), with modifications specific to the North Carolina Trauma Registry. It is to be used in lieu of the NTRACS data It provides a brief summary of every data point used in North Carolina, and notes where there are custom options standard throughout the State. It does not cover those data items that are customized or by each site specifically and not used statewide. Some data points are not downloaded to the State, i.e., the Central Data Collection Agency. These datapoints are noted with a "d" in the Download Scenario column. Therefore, the statewide registry does not include these data points, although each individual hospital has them. The column labeled Download Scenario contains information on whether datapoints are to be downloaded to the Central Data Collection Agency (the State) and whether datapoints are sent to the National Trauma Data Bank (NTDB). This column contains one of three values: h d: This variable is not to be downloaded to the State and is not sent to NTDB h s: This variable is to be downloaded to the State, but the data are not sent to NTDB h s,n: This variable is to be downloaded to the State, and may be forwarded to the NTDB. For the NC Custom Data Points, field type and size information have been included in the Definitions column. -
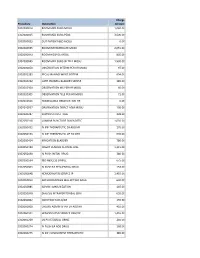
Procedure Description Charge Amount 0302000014 ROOM MED
Charge Procedure Description Amount 0302000014 ROOM MED SURG MOSU 1,040.00 0302000015 ROOM MED SURG PEDS 3,500.00 0302000033 OUT PATIENT BED MOSU 0.00 0302000035 ROOM INTERMEDIATE MOSU 2,055.00 0302000043 ROOM HOSPICE MOSU 805.00 0302000045 ROOM MED SURG W TELE MOSU 1,530.00 0302000050 OBSERVATION INTERM PER HR MOSU 97.00 0302002283 FECAL MANAGEMENT SYSTEM 694.00 0302010232 CATH INDWELL BLADDER SIMPLE 185.00 0302010304 OBSERVATION MS PER HR MOSU 60.00 0302010305 OBSERVATION TELE PER HR MOSU 71.00 0302010556 NONBILLABLE OBSERVATION HR 0.00 0302010557 OBSERVATION DIRECT ADM MOSU 130.00 0302020287 SUPPLIES CHEST TUBE 228.00 0302050318 LUMBAR PUNCTURE DIAGNOSTIC 1,035.00 0302050451 IV INF THERAPEUTIC EA ADD HR 170.00 0302050453 IV INF THERAPEUTIC UP TO 1HR 635.00 0302050454 IRRIGATION BLADDER 780.00 0302050480 INSERT VENOUS CENTRAL LINE 1,315.00 0302050490 IV PUSH INITIAL DRUG 380.00 0302050534 I&D ABSCESS SIMPLE 675.00 0302050603 IV PUSH EA SEQUENTIAL DRUG 153.00 0302050648 HEMODIALYSIS SERVICE IP 1,455.00 0302050813 ARTHROCENTESIS MAJ JNT WO IMAG 630.00 0302050885 ADMIN IMMUNIZATION 145.00 0302050948 DIALYSIS INTRAPERITONEAL SERV 655.00 0302060002 INJECTION SUB-Q/IM 155.00 0302060008 CHEMO ADMIN IV INF EA ADD HR 410.00 0302060101 HEMODIALYSIS SERVICE OBS/OP 1,455.00 0302060269 US PV RESIDUAL URINE 240.00 0302060274 IV PUSH EA ADD DRUG 168.00 0302060275 IV INF CONCURRENT THERAPEUTIC 385.00 0302060276 IV INF SEQUENTIAL THER UP TO 1 191.00 0302060293 INSERT STRAIGHT CATH THERAPEUT 185.00 0302060372 CHEMO ADMIN IV INF SEQ 1 HR 525.00 0302060373 -

Annual Report for 2017 Was Sent out to All the Member Organizations and Will Be Available on the CAMTS Website
Year 2018 4124 Clemson Blvd, Anderson, SC 29621 www.camts.org O -864 287-4177 From the Executive Director The year 2018 was a busy year of expansion and consolidation. For example, we accredited 58 medical transport services. This does not seem like a large number but because we often have many sites combined under the same program’s survey – these 58 programs included 271 bases. For initial accreditation site visits to new applicants, this means we visit every base. For reaccreditations, we will visit new or changed bases and usually include an unannounced base visit. This involves a great deal of planning, logistics, and travel to remote bases with as many as 5 site surveyors at times. Gigi Randall, our Administrative Assistant, and our experienced lead site surveyors do a wonderful job of scheduling and coordinating these visits. We changed the policy last year to accommodate these large services under one owner/operator. Combined services may apply as one service if they have the same mission, management, policies, medical direction and protocols, and a common Part 135 certificate. Shelley Dixon, our bookkeeper assistant, keeps the new and reaccrediting services represented correctly and promptly on the camts.org website. In addition to complex site surveys, we completed the standards and process for Special Operations – Medical Retrieval which were accepted by both CAMTS and CAMTS EU Board of Directors and is available worldwide. Special Operations – Medical Retrieval includes criteria for services that provide tactical rescue or “SWAT: call-outs and citizen recovery from potentially unstable environments. There are already 2 applications for this specific accreditation. -

Vidant Stroke Care
Ashley Elks BSN, RN, PCCN Director Stroke and Neuroscience Vidant Medical Center Greenville, NC Our mission To improve the health and well-being of eastern North Carolina To enhance the quality of life for the people Our visionand communitiesTo become we serve, the touch national and supportmodel for rural health and wellness by creating a VISIONpremier, trusted health care delivery and education system Where incredible people provide incredible care… every day Our values Integrity VALUESCompassion Excellence…Education our standard Compassion…Accountability our distinction Teamwork… our advantage Education…Safety our investment Innovation…Teamwork our future 2 COPYRIGHT 2015 VIDANT HEALTH Vidant Health • Not-for-profit hospital system • Serves more than 1.4 million people in 29 eastern North Carolina • Health system comprised of 8 hospitals (9 w/ addition of Halifax) • Vidant Medical Center is the hub 3 COPYRIGHT 2015 VIDANT HEALTH Vidant Medical Center • Greenville, NC • > 900 bed hospital • Level 1 trauma center • Comprehensive Stroke Center • Regional referral hospital for the eastern 1/3 of NC • Magnet® Facility • Partnership with East Carolina University – Brody School of Medicine and College of Nursing 4 COPYRIGHT 2015 VIDANT HEALTH Buckle of the Stroke Belt • The coastal plain of North Carolina is in the nation’s “Stroke Buckle” • Death rate from stroke is twice as high as the national average Stroke Deaths per 100,000 Source: CDC Interactive Atlas of Heart Disease 5 2013-2015 COPYRIGHT 2015 VIDANT HEALTH Buckle of the Stroke Belt -

Pharmaceuticals As Environmental Contaminants
PharmaceuticalsPharmaceuticals asas EnvironmentalEnvironmental Contaminants:Contaminants: anan OverviewOverview ofof thethe ScienceScience Christian G. Daughton, Ph.D. Chief, Environmental Chemistry Branch Environmental Sciences Division National Exposure Research Laboratory Office of Research and Development Environmental Protection Agency Las Vegas, Nevada 89119 [email protected] Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Why and how do drugs contaminate the environment? What might it all mean? How do we prevent it? Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada This talk presents only a cursory overview of some of the many science issues surrounding the topic of pharmaceuticals as environmental contaminants Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada A Clarification We sometimes loosely (but incorrectly) refer to drugs, medicines, medications, or pharmaceuticals as being the substances that contaminant the environment. The actual environmental contaminants, however, are the active pharmaceutical ingredients – APIs. These terms are all often used interchangeably Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Office of Research and Development Available: http://www.epa.gov/nerlesd1/chemistry/pharma/image/drawing.pdfNational -

2015 Pitt County Community Health Needs Assessment
PITT COUNTY Community Health Needs Assessment 2015 Table of Contents Acknowledgements ........................................................................................................................... 5 1 NO R T H CAROLINA Table of Contents Acknowledgements ……………………………………………………………………………………………………………………………. 5 Executive Summary ……………………………………………………………………………………………………………………………. 7 Community Health Needs Assessment Background and Purpose …………………………….……………………… 10 Team Composition …………………………………………………………………………………………………………………….……… 10 Data Collection Process ………………………………………………………………………………………………………………….…. 10 Health Priorities Selection …………………………………………………………………………………………………………………. 11 County Overview ………………………………………………………………………………………………………………………………. 13 Demographics Population Estimates ………………………………………………………………………………………………………………………… 13 Age Distribution ………………………………………………………………………………………………………………………………… 14 Race and Ethnicity……………………………………………………………………………………………………………………………… 14 Education …………………………………………………………………………………………………………………………………………. 15 Economic Factors Income and Poverty ……………………………………………………………………………………………………………………….... 15 Employment……………………………………………………………………………………………………………………………………… 17 Homeownership……………………………………………………………………………………………………………………………….. 17 Agriculture………………………………………………………………………………………………………………………………………... 17 Transportation…………………………………………………………………………………………………………………………………… 17 Crime and Intentional Injuries………………………………………………………………………………………………………….. 18 Leading Causes of Death……………………………………………………………………………………………………………………. 19 Leading -

2019 Annual Report
ANNUAL REPORT 2019 Year 2019 4124 Clemson Blvd, Anderson, SC 29621 www.camts.org O -864 287-4177 From the Executive Director The year 2019 was a busy year overall. We accredited 62 medical transport services and conducted the first site visit for a Special Operations – Medical retrieval service. The Special Operations standards were developed with input from ex-military and rescue experts to address citizen recovery operations that are often contracted by the government to assist military and paramilitary operations in remote parts of the world. The standards were approved by both CAMTS and CAMTS Global boards of directors. CAMTS and CAMTS Global have joint meetings each July. We added Dr. Dhun Damrongsak, the medical director from BDMS Emergency Medical Services in Bangkok to the CAMTS Global board this year as an ad hoc member. BDMS Emergency Medical Services is a dual accredited service (CAMTS-CAMTS Global) offering rotor wing, fixed wing, surface and marine transport in Thailand and surrounding countries in Asia. I had the honor of speaking at the BDMS annual conference in August. The title of my presentation was “Medical Transport Standards and Expansion into Asia” (pictured on page 17 of this report). We changed the reporting for Safety Culture Survey results to the programs this year. The results are now reported in graphs that compare the six cultures that impact the program’s overall safety culture. The chart also compares the program’s results to the average results of other programs received to date. In the future, programs will be able to compare results from the previous suite visit with current results as well. -

US8074644.Pdf
USOO8074644B2 (12) United States Patent (10) Patent No.: US 8,074,644 B2 Hale et al. (45) Date of Patent: *Dec. 13, 2011 (54) METHOD OF FORMING AN AEROSOL FOR (56) References Cited NHALATION DELVERY (75) Inventors: Ron L. Hale, Sandia Park, NM (US); U.S. PATENT DOCUMENTS Craig C. Hodges, Walnut Creek, CA 1,239,634 A 9, 1917 Stuart (US); Peter M. Lloyd, Walnut Creek, CA (US); Daniel Mufson, Napa, CA (Continued) (US); Daniel D. Rogers, Oakland, CA (US); Soonho Song, Hillsborough, CA FOREIGN PATENT DOCUMENTS (US); Martin J. Wensley, Los Gatos, CA 2152684 1, 1996 CA (US); Daniel J. Myers, Mountain (Continued) View, CA (US); Jeffrey A. McKinney, Lafayette, CA (US); Reynaldo J. OTHER PUBLICATIONS Quintana, Redwood City, CA (US); U.S. Appl. No. 1 1/687,466, filed Mar. 16, 2007, Zaffaroni et al. Joshua D. Rabinowitz, Princeton, NJ (US) (Continued) (73) Assignee: Alexza Pharmaceuticals, Inc., Primary Examiner — Steven Douglas Mountain View, CA (US) (74) Attorney, Agent, or Firm — Swanson & Bratschun, (*) Notice: Subject to any disclaimer, the term of this L.L.C. patent is extended or adjusted under 35 (57) ABSTRACT U.S.C. 154(b) by 272 days. The present invention relates to the inhalation delivery of This patent is Subject to a terminal dis aerosols containing Small particles. Specifically, it relates to a claimer. method of forming an aerosol for use in inhalation therapy. In (21) Appl. No.: 12/471,070 a method aspect of the present invention, a method of forming (22) Filed: May 22, 2009 an aerosol for use in inhalation therapy is provided. -

2000 Dialysis of Drugs
2000 Dialysis of Drugs PROVIDED AS AN EDUCATIONAL SERVICE BY AMGEN INC. I 2000 DIAL Dialysis of Drugs YSIS OF DRUGS Curtis A. Johnson, PharmD Member, Board of Directors Nephrology Pharmacy Associates Ann Arbor, Michigan and Professor of Pharmacy and Medicine University of Wisconsin-Madison Madison, Wisconsin William D. Simmons, RPh Senior Clinical Pharmacist Department of Pharmacy University of Wisconsin Hospital and Clinics Madison, Wisconsin SEE DISCLAIMER REGARDING USE OF THIS POCKET BOOK DISCLAIMER—These Dialysis of Drugs guidelines are offered as a general summary of information for pharmacists and other medical professionals. Inappropriate administration of drugs may involve serious medical risks to the patient which can only be identified by medical professionals. Depending on the circumstances, the risks can be serious and can include severe injury, including death. These guidelines cannot identify medical risks specific to an individual patient or recommend patient treatment. These guidelines are not to be used as a substitute for professional training. The absence of typographical errors is not guaranteed. Use of these guidelines indicates acknowledgement that neither Nephrology Pharmacy Associates, Inc. nor Amgen Inc. will be responsible for any loss or injury, including death, sustained in connection with or as a result of the use of these guidelines. Readers should consult the complete information available in the package insert for each agent indicated before prescribing medications. Guides such as this one can only draw from information available as of the date of publication. Neither Nephrology Pharmacy Associates, Inc. nor Amgen Inc. is under any obligation to update information contained herein. Future medical advances or product information may affect or change the information provided.