Appendix A.2: Tier 2 Surgical Procedure Terms and Definitions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidelines on the Diagnosis and Management of Pericardial
European Heart Journal (2004) Ã, 1–28 ESC Guidelines Guidelines on the Diagnosis and Management of Pericardial Diseases Full Text The Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology Task Force members, Bernhard Maisch, Chairperson* (Germany), Petar M. Seferovic (Serbia and Montenegro), Arsen D. Ristic (Serbia and Montenegro), Raimund Erbel (Germany), Reiner Rienmuller€ (Austria), Yehuda Adler (Israel), Witold Z. Tomkowski (Poland), Gaetano Thiene (Italy), Magdi H. Yacoub (UK) ESC Committee for Practice Guidelines (CPG), Silvia G. Priori (Chairperson) (Italy), Maria Angeles Alonso Garcia (Spain), Jean-Jacques Blanc (France), Andrzej Budaj (Poland), Martin Cowie (UK), Veronica Dean (France), Jaap Deckers (The Netherlands), Enrique Fernandez Burgos (Spain), John Lekakis (Greece), Bertil Lindahl (Sweden), Gianfranco Mazzotta (Italy), Joa~o Morais (Portugal), Ali Oto (Turkey), Otto A. Smiseth (Norway) Document Reviewers, Gianfranco Mazzotta, CPG Review Coordinator (Italy), Jean Acar (France), Eloisa Arbustini (Italy), Anton E. Becker (The Netherlands), Giacomo Chiaranda (Italy), Yonathan Hasin (Israel), Rolf Jenni (Switzerland), Werner Klein (Austria), Irene Lang (Austria), Thomas F. Luscher€ (Switzerland), Fausto J. Pinto (Portugal), Ralph Shabetai (USA), Maarten L. Simoons (The Netherlands), Jordi Soler Soler (Spain), David H. Spodick (USA) Table of contents Constrictive pericarditis . 9 Pericardial cysts . 13 Preamble . 2 Specific forms of pericarditis . 13 Introduction. 2 Viral pericarditis . 13 Aetiology and classification of pericardial disease. 2 Bacterial pericarditis . 14 Pericardial syndromes . ..................... 2 Tuberculous pericarditis . 14 Congenital defects of the pericardium . 2 Pericarditis in renal failure . 16 Acute pericarditis . 2 Autoreactive pericarditis and pericardial Chronic pericarditis . 6 involvement in systemic autoimmune Recurrent pericarditis . 6 diseases . 16 Pericardial effusion and cardiac tamponade . -

Surgical Management of Transcatheter Heart Valves
Corporate Medical Policy Surgical Management of Transcatheter Heart Valves File Name: surgica l_management_of_transcatheter_heart_valves Origination: 1/2011 Last CAP Review: 6/2021 Next CAP Review: 6/2022 Last Review: 6/2021 Description of Procedure or Service As the proportion of older adults increases in the U.S. population, the incidence of degenerative heart valve disease also increases. Aortic stenosis and mitra l regurgita tion are the most common valvular disorders in adults aged 70 years and older. For patients with severe valve disease, heart valve repair or replacement involving open heart surgery can improve functional status and qua lity of life. A variety of conventional mechanical and bioprosthetic heart valves are readily available. However, some individuals, due to advanced age or co-morbidities, are considered too high risk for open heart surgery. Alternatives to the open heart approach to heart valve replacement are currently being explored. Transcatheter heart valve replacement and repair are relatively new interventional procedures involving the insertion of an artificial heart valve or repair device using a catheter, rather than through open heart surgery, or surgical valve replacement (SAVR). The point of entry is typically either the femoral vein (antegrade) or femora l artery (retrograde), or directly through the myocardium via the apical region of the heart. For pulmonic and aortic valve replacement surgery, an expandable prosthetic heart valve is crimped onto a catheter and then delivered and deployed at the site of the diseased native valve. For valve repair, a small device is delivered by catheter to the mitral valve where the faulty leaflets are clipped together to reduce regurgitation. -
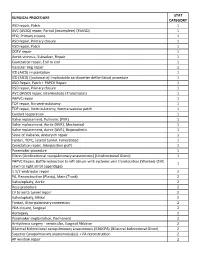
Surgeries by STAT Category
STAT SURGICAL PROCEDURE CATEGORY ASD repair, Patch 1 AVC (AVSD) repair, Partial (Incomplete) (PAVSD) 1 PFO, Primary closure 1 ASD repair, Primary closure 1 VSD repair, Patch 1 DCRV repair 1 Aortic stenosis, Subvalvar, Repair 1 Coarctation repair, End to end 1 Vascular ring repair 1 ICD (AICD) implantation 1 ICD (AICD) ([automatic] implantable cardioverter deFibrillator) procedure 1 ASD Repair, Patch + PAPCV Repair 1 VSD repair, Primary closure 1 AVC (AVSD) repair, Intermediate (Transitional) 1 PAPVC repair 1 TOF repair, No ventriculotomy 1 TOF repair, Ventriculotomy, Nontransanular patch 1 Conduit reoperation 1 Valve replacement, Pulmonic (PVR) 1 Valve replacement, Aortic (AVR), Mechanical 1 Valve replacement, Aortic (AVR), Bioprosthetic 1 Sinus oF Valsalva, Aneurysm repair 1 Fontan, TCPC, Lateral tunnel, Fenestrated 1 Coarctation repair, Interposition graFt 1 Pacemaker procedure 1 Glenn (Unidirectional cavopulmonary anastomosis) (Unidirectional Glenn) 1 PAPVC Repair, BaFFle redirection to leFt atrium with systemic vein translocation (Warden) (SVC 1 sewn to right atrial appendage) 1 1/2 ventricular repair 2 PA, Reconstruction (Plasty), Main (Trunk) 2 Valvuloplasty, Aortic 2 Ross procedure 2 LV to aorta tunnel repair 2 Valvuloplasty, Mitral 2 Fontan, Atrio-pulmonary connection 2 PDA closure, Surgical 2 Aortopexy 2 Pacemaker implantation, Permanent 2 Arrhythmia surgery - ventricular, Surgical Ablation 2 Bilateral bidirectional cavopulmonary anastomosis (BBDCPA) (Bilateral bidirectional Glenn) 2 Superior Cavopulmonary anastomosis(es) + PA -

Valve Repair for Mitral Insufficiency Without Stenosis Richard B
Journal of Insurance Medicine Volume 20, No. I 1988 Mortality Abstract Valve Repair for Mitral Insufficiency Without Stenosis Richard B. Singer, M.D. Consultant Association of Life Insurance Medical Directors of America References: (1) JW Kirklin, ’WIitral Valve Repair for Mitral those with isolated MVR, with or without tricuspid an- Incompetence." Mod Concepts of CV Dis, 56:7-11 (Feb. nuloplasty. The highest perioperative rate of 11.1% was ex- 1987). perienced in the group with associated CBPS, and the rate (2) ALIMDA and Society of Actuaries, ’~¢Iedical was nearly as high when the associated surgery was aortic Risks 1987: Analysis of Mortality and Survival." In final valve replacement. preparation (1988). Despite the complete lack of age/sex information, the author Subjects Studied: A series of 210 patients diagnosed at the has provided an expected survival curve based on "an age- Medical Center of the University of Alabama at Birmingham, sex-race-matched general population," but the source tables 1967-1985 as having mitral insufficiency without stenosis, are not cited. From this curve it has been possible to derive and treated by surgical repair of the mitral valve (MVR) in- and graduate expected annual rates, q/or ~/, as shown in stead of mitral valve replacement. The largest group in the Table B. Although the derivation is reasonably accurate for series consisted of 86 patients with isolated MVR, although the first year, the reader should be cautioned that the an- a few of these also had tricuspid valve annuloplasty. The nual increase of about 8% per year in qZ may be much too remaining patients had associated cardiac surgery: 63 patients high as compared with an increase of about 1% per year with coronary bypass (CBPS); 31 patients with aortic valve found for qZ, all ages combined, from data by age group replacement (AVR); 27 patients with repair of a congenital and sex for a series of patients with mitral valve replace- cardiac defect; two patients with pericardiectomy and one ment in one of the abstracts in reference 2. -

Note to Users for Pedcsrs 2010 Data Collection
Note to Users for PedCSRS 2010 Data Collection The PedCSRS coding instructions and data element definitions issued for 2009 discharges will remain in effect for 2010 discharges. There are no changes to the data collection form, definitions or coding instructions. The attached document contains the current procedure codes that should be used for 2010 PedCSRS reporting. This includes the changes to procedure codes made for July 2009 Adult CSRS reporting. For discharge year 2010, use form DOH-2254p (1/09). Although the bottom of that form states “2009 Discharges,” the effective period for this form has been extended through December 2010 discharges. The file specification for 2010 PedCSRS data contains no changes from the 2009 file structure. The document has been updated only for dates and naming conventions. A copy of the current PedCSRS file specifications document can be obtained from the Cardiac Services Program. Reporting Schedule Pediatric CSRS data is reported quarterly by discharge date. It is due to the Cardiac Services Program two months after the end of the quarter. The 2010 reporting schedule is as follows. Quarter 1 (1/1/10 – 3/31/10 Discharges) due on or before May 31, 2010 Quarter 2 (4/1/10 – 6/30/10 Discharges) due on or before August 31, 2010 Quarter 3 (7/1/10 – 9/30/10 Discharges) due on or before November 30, 2010 Quarter 4 (10/1/10 – 12/31/10 Discharges) due on or before February 28, 2011 Limited extensions to the above deadlines will be granted on a case by case basis when warranted by extenuating circumstances. -

Surgery for Acquired Heart Disease
View metadata, citation and similar papers at core.ac.uk brought to you byCORE provided by Elsevier - Publisher Connector SURGERY FOR ACQUIRED HEART DISEASE EARLY RESULTS WITH PARTIAL LEFT VENTRICULECTOMY Patrick M. McCarthy, MD a Objective: We sought to determine the role of partial left ventriculectomy in Randall C. Starling, MD b patients with dilated cardiomyopathy. Methods: Since May 1996 we have James Wong, MBBS, PhD b performed partial left ventriculectomy in 53 patients, primarily (94%) in Gregory M. Scalia, MBBS b heart transplant candidates. The mean age of the patients was 53 years Tiffany Buda, RN a Rita L. Vargo, MSN, RN a (range 17 to 72 years); 60% were in class IV and 40% in class III. Marlene Goormastic, MPH c Preoperatively, 51 patients were thought to have idiopathic dilated cardio- James D. Thomas, MD b myopathy, one familial cardiomyopathy, and one valvular cardiomyopathy. Nicholas G. Smedira, MD a As our experience accrued we increased the extent of left ventriculectomy James B. Young, MD b and more complex mitral valve repairs. For two patients mitral valve replacement was performed. For 51 patients the anterior and posterior mitral valve leaflets were approximated (Alfieri repair); 47 patients also had ring posterior annuloplasty. In 27 patients (5!%) one or both papillary muscles were divided, additional left ventricular wall was resected, and the papillary muscle heads were reimplanted. Results: Echocardiography showed a significant decrease in left ventricular dimensions after resection (8.3 cm to 5.8 cm), reduction in mitral regurgitation (2.8+ to 0), and increase in forward ejection fraction (15.7% to 32.7%). -

Mustard Procedure Br Heart J: First Published As 10.1136/Hrt.72.1.85 on 1 July 1994
Br Heart J 1994;72:85-88 85 Transcatheter stent implantation for recurrent pulmonary venous pathway obstruction after the Mustard procedure Br Heart J: first published as 10.1136/hrt.72.1.85 on 1 July 1994. Downloaded from Martin C K Hosking, Kenneth A Murdison, Walter J Duncan Abstract pathway obstruction reaching a maximum A 5 year old boy presented with obstruc- flow velocity of 2'0 m/s associated with a tion of the pulmonary venous pathway stenosis measuring 2-3 mm in diameter. four years after the Mustard procedure. Right ventricular dysfunction was a persistent A successful balloon dilatation ofthe pul- echocardiographic finding associated with an monary venous pathway was performed increase in left ventricle dimension in but the benefit was transient. Placement response to the rise of pulmonary arterial of a 10 mm balloon expandable intravas- pressure secondary to the pulmonary venous cular stent across the recurrent stenosis inflow obstruction. A significant systemic to resulted in complete relief ofthe obstruc- pulmonary venous chamber baffle leak mea- tion with prompt resolution ofthe clinical suring 5 mm x 4 mm with right to left shunting signs. The delivery system was modified was seen by Doppler colour flow mapping and to facilitate stent delivery. contributed to central cyanosis. Serial chest x ray films showed persistence of cardiomegaly (Br Heart 1994;72:85-88) with increasing evidence of pulmonary venous congestion. To avoid the risks of surgical repair in a Paediatric experience with transcatheter patient with impaired -

Arterial Switch Operation Surgery Surgical Solutions to Complex Problems
Pediatric Cardiovascular Arterial Switch Operation Surgery Surgical Solutions to Complex Problems Tom R. Karl, MS, MD The arterial switch operation is appropriate treatment for most forms of transposition of Andrew Cochrane, FRACS the great arteries. In this review we analyze indications, techniques, and outcome for Christian P.R. Brizard, MD various subsets of patients with transposition of the great arteries, including those with an intact septum beyond 21 days of age, intramural coronary arteries, aortic arch ob- struction, the Taussig-Bing anomaly, discordant (corrected) transposition, transposition of the great arteries with left ventricular outflow tract obstruction, and univentricular hearts with transposition of the great arteries and subaortic stenosis. (Tex Heart Inst J 1997;24:322-33) T ransposition of the great arteries (TGA) is a prototypical lesion for pediat- ric cardiac surgeons, a lethal malformation that can often be converted (with a single operation) to a nearly normal heart. The arterial switch operation (ASO) has evolved to become the treatment of choice for most forms of TGA, and success with this operation has become a standard by which pediatric cardiac surgical units are judged. This is appropriate, because without expertise in neonatal anesthetic management, perfusion, intensive care, cardiology, and surgery, consistently good results are impossible to achieve. Surgical Anatomy of TGA In the broad sense, the term "TGA" describes any heart with a discordant ven- triculoatrial (VA) connection (aorta from right ventricle, pulmonary artery from left ventricle). The anatomic diagnosis is further defined by the intracardiac fea- tures. Most frequently, TGA is used to describe the solitus/concordant/discordant heart. -

Reduction Ventriculoplasty for Dilated Cardiomyopathy : the Batista Procedure Shahram Salemy Yale University
Yale University EliScholar – A Digital Platform for Scholarly Publishing at Yale Yale Medicine Thesis Digital Library School of Medicine 1999 Reduction ventriculoplasty for dilated cardiomyopathy : the Batista procedure Shahram Salemy Yale University Follow this and additional works at: http://elischolar.library.yale.edu/ymtdl Recommended Citation Salemy, Shahram, "Reduction ventriculoplasty for dilated cardiomyopathy : the Batista procedure" (1999). Yale Medicine Thesis Digital Library. 3123. http://elischolar.library.yale.edu/ymtdl/3123 This Open Access Thesis is brought to you for free and open access by the School of Medicine at EliScholar – A Digital Platform for Scholarly Publishing at Yale. It has been accepted for inclusion in Yale Medicine Thesis Digital Library by an authorized administrator of EliScholar – A Digital Platform for Scholarly Publishing at Yale. For more information, please contact [email protected]. SlDDCITOM VENTRICULOPIASTy FOR DILATED CARDIOMYOPATHY THE BATISTA PROCEDURE W«M * (e,yx»> ShaLramSalemy YALE DNIVERSriY YALE UNIVERSITY CUSHING/WHITNEY MEDICAL LIBRARY Permission to photocopy or microfilm processing of this thesis for the purpose of individual scholarly consultation or reference is hereby granted by the author. This permission is not to be interpreted as affecting publication of this work or otherwise placing it in the public domain, and the author reserves all rights of ownership guaranteed under common law protection of unpublished manuscripts. Signature of Author Date REDUCTION VENTRICULOPLASTY FOR DILATED CARDIOMYOPATHY: THE BATISTA PROCEDURE Shahram Salemy B.S., George Tellides M.D., Ph.D., and John A. Elefteriades M.D. February 5, 1999 r 113 f'Uh (e(e.cl 0 REDUCTION VENTRICULOPLASTY FOR DILATED CARDIOMYOPATHY: THE BATISTA PROCEDURE. -

Mustard Operation
Mustard Operation William G. Williams The natural history of infants with complete transposi- desaturation. Mustard reasoned that both vena cavae tion (TGA) is rapid deterioration and death within the could be deliberately diverted to the left atrium while first few months of life. The few babies who do survive allowing the pulmonary return to enter the right atrium, beyond the first year of life usually have an associated thereby correcting the circulation in patients with TGA. ventricular septal defect (VSD) and subsequently dete- The Mustard baffle operation was a simple, reproduc- riorate with progressive pulmonary vascular disease. ible, and elegant technique of transposing venous re- Intervention to improve the outlook for newborns turn. It was easier to learn than the Senning operation, with TGA began with surgical resection of the atrial and became the standard repair in most of the world. septum.' The enlarged atrial defect improves mixing of the pulmonary and systemic circulations, thereby in- creasing the systemic oxygen saturation. Balloon atrial Indications for the Mustard Operation in 1998 septostomy, as described by Rashkind and Miller in The atrial repair of TGA has been almost entirely 1966,2 replaced surgical septectomy. The balloon cath- replaced by the arterial switch operation. However, eter technique continues to provide effective emergency there are three situations were the Mustard operation palliation for the newborn with TGA. may be indicated: In the early 1950s, a number of surgeons, including 1. For infants with isolated TGA who first present M~stard,~attempted arterial repair of TGA in infants, after the neonatal period, the Mustard operation is an all without success until Jatene's report in 1978.4 In alternative to the two-stage arterial switch operation, 1954, Albert5 proposed a surgical technique to trans- ie, a preliminary pulmonary artery banding to prepare pose the venous inflow within the atria to correct TGA. -

Long-Term Outcomes of the Neoaorta After Arterial Switch Operation for Transposition of the Great Arteries Jennifer G
ORIGINAL ARTICLES: CONGENITAL HEART SURGERY CONGENITAL HEART SURGERY: The Annals of Thoracic Surgery CME Program is located online at http://cme.ctsnetjournals.org. To take the CME activity related to this article, you must have either an STS member or an individual non-member subscription to the journal. CONGENITAL HEART Long-Term Outcomes of the Neoaorta After Arterial Switch Operation for Transposition of the Great Arteries Jennifer G. Co-Vu, MD,* Salil Ginde, MD,* Peter J. Bartz, MD, Peter C. Frommelt, MD, James S. Tweddell, MD, and Michael G. Earing, MD Department of Pediatrics, Division of Pediatric Cardiology, and Department of Internal Medicine, Division of Cardiovascular Medicine, and Department of Cardiothoracic Surgery, Medical College of Wisconsin, Milwaukee, Wisconsin Background. After the arterial switch operation (ASO) score increased at an average rate of 0.08 per year over for transposition of the great arteries (TGA), the native time after ASO. Freedom from neoaortic root dilation at pulmonary root and valve function in the systemic posi- 1, 5, 10, and 15 years after ASO was 84%, 67%, 47%, and tion, and the long-term risk for neoaortic root dilation 32%, respectively. Risk factors for root dilation include -pre ,(0.003 ؍ and valve regurgitation is currently undefined. The aim history of double-outlet right ventricle (p and length of ,(0.01 ؍ of this study was to determine the prevalence and pro- vious pulmonary artery banding (p Neoaortic valve regurgitation of at .(0.04 ؍ gression of neoaortic root dilation and neoaortic valve follow-up (p regurgitation in patients with TGA repaired with the least moderate degree was present in 14%. -

Positive Maternal and Foetal Outcomes After Cardiopulmonary Bypass Surgery
Case Study: Positive maternal and foetal outcomes after cardiopulmonary bypass surgery Positive maternal and foetal outcomes after cardiopulmonary bypass surgery in a parturient with severe mitral valve disease aMokgwathi GT, MBChB aLebakeng EM, MBChB, DA(SA), MMed(Anaes) bOgunbanjo GA, MBBS, FCFP(SA), MFamMed, FACRRM, FACTM, FAFP(SA), FWACP(Fam Med) aDepartment of Anaesthesiology, University of Limpopo (Medunsa Campus) bDepartment of Family Medicine and Primary Health Care, University of Limpopo (Medunsa Campus) Correspondence to: Dr GT Mokgwathi, e-mail: [email protected] Keywords: anaesthesia, cardiac surgery, parturient, cardiopulmonary bypass surgery, mitral valve replacement Abstract This case study describes the successful management of a parturient with severe mitral stenosis and moderate mitral regurgitation who underwent cardiopulmonary bypass (CPB) surgery. A healthy baby was delivered by Caesarean section 11 days later. The effects of CPB surgery and mitral valve replacement on parturient and foetus are discussed. Peer reviewed. (Submitted: 2011-01-04, Accepted: 2011-06-16) © SASA South Afr J Anaesth Analg 2011;17(4):299-302 Introduction she was classified as New York Heart Association (NYHA) class III and World Health Organization (WHO) heart Heart disease is the primary cause of nonobstetric mortality failure stage C. Her blood pressure was 90/60 mmHg in pregnancy, occurring in 1-4 % of pregnancies1-3 and and her heart rate was 82 beats per minute and regular. accounting for 10–15 % of maternal mortality in developed She had a tapping apex beat, loud first heart sound, loud countries.1,2 Cardiac disease contributes to 40.2% of pulmonary component of the second heart sound and a maternal deaths in South Africa.4 Soma-Pillay et al noted grade 3/4 diastolic murmur heard loudest at the apex.