<I>Ameerega Trivittata</I>
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tadpole Consumption Is a Direct Threat to the Endangered Purple Frog, Nasikabatrachus Sahyadrensis
SALAMANDRA 51(3) 252–258 30 October 2015 CorrespondenceISSN 0036–3375 Correspondence Tadpole consumption is a direct threat to the endangered purple frog, Nasikabatrachus sahyadrensis Ashish Thomas & S. D. Biju Systematics Lab, Department of Environmental Studies, University of Delhi, Delhi 110 007, India Corresponding author: S. D. Biju, e-mail: [email protected] Manuscript received: 5 July 2014 Accepted: 30 December 2014 by Alexander Kupfer Amphibians across the world are suffering alarming popu- Southeast Asia have witnessed drastic population declines lation declines with nearly one third of the ca 7,300 species caused by overexploitation over the last couple of decades being threatened worldwide (Stuart et al. 2008, Wake & (Warkentin et al. 2008). Often, natural populations are Vredenburg 2008, IUCN 2014). Major factors attributed harvested without regard of the consequences or implica- to the decline include habitat destruction (Houlahan et tions of this practice on the dynamics or sustainability of al. 2000, Sodhi et al. 2008), chemical pollution (Ber ger the exploited populations (Getz & Haight 1989). When 1998), climate change (Adams 1999, Carpenter & Tur- the extent of exploitation is greater than the sustaining ca- ner 2000), diseases (McCallum 2007, Cushman 2006), pacity or turnover rate of a species, there is every possi- and invasive species (Boone & Bridges 2003). The West- bility that the species may become locally extinct, which ern Ghats of India, a global hotspot for amphibian diver- would subsequently have drastic ecological implications sity and endemism (Biju 2001, Biju & Bossuyt 2003), has in the particular region (Duffy 2002, Wright et al. 2006, more than 40% of its amphibian fauna threatened with ex- Carpenter et al. -

What Do Tadpoles Really Eat? Assessing the Trophic Status of an Understudied and Imperiled Group of Consumers in Freshwater Habitats
Freshwater Biology (2007) 52, 386–395 doi:10.1111/j.1365-2427.2006.01694.x OPINION What do tadpoles really eat? Assessing the trophic status of an understudied and imperiled group of consumers in freshwater habitats RONALD ALTIG,* MATT R. WHILES† AND CINDY L. TAYLOR‡ *Department of Biological Sciences, Mississippi State University, Mississippi State, MS, U.S.A. †Department of Zoology and Center for Ecology, Southern Illinois University, Carbondale, IL, U.S.A. ‡Department of Biology, Austin Peay State University, Clarksville, TN, U.S.A. SUMMARY 1. Understanding the trophic status of consumers in freshwater habitats is central to understanding their ecological roles and significance. Tadpoles are a diverse and abundant component of many freshwater habitats, yet we know relatively little about their feeding ecology and true trophic status compared with many other consumer groups. While many tadpole species are labelled herbivores or detritivores, there is surprisingly little evidence to support these trophic assignments. 2. Here we discuss shortcomings in our knowledge of the feeding ecology and trophic status of tadpoles and provide suggestions and examples of how we can more accurately quantify their trophic status and ecological significance. 3. Given the catastrophic amphibian declines that are ongoing in many regions of the planet, there is a sense of urgency regarding this information. Understanding the varied ecological roles of tadpoles will allow for more effective conservation of remaining populations, benefit captive breeding programmes, and allow for more accurate predic- tions of the ecological consequences of their losses. Keywords: amphibian, assimilation, diet, feeding behaviour, omnivory Amphibians are disappearing from the planet at an of the functional roles and trophic status of general- alarming rate (Stuart et al., 2004; Lips et al., 2005). -

A New Species of the Genus Nasikabatrachus (Anura, Nasikabatrachidae) from the Eastern Slopes of the Western Ghats, India
Alytes, 2017, 34 (1¢4): 1¢19. A new species of the genus Nasikabatrachus (Anura, Nasikabatrachidae) from the eastern slopes of the Western Ghats, India S. Jegath Janani1,2, Karthikeyan Vasudevan1, Elizabeth Prendini3, Sushil Kumar Dutta4, Ramesh K. Aggarwal1* 1Centre for Cellular and Molecular Biology (CSIR-CCMB), Uppal Road, Tarnaka, Hyderabad, 500007, India. <[email protected]>, <[email protected]>. 2Current Address: 222A, 5th street, Annamalayar Colony, Sivakasi, 626123, India.<[email protected]>. 3Division of Vertebrate Zoology, Department of Herpetology, American Museum of Natural History, Central Park West at 79th Street, New York NY 10024-5192, USA. <[email protected]>. 4Nature Environment and Wildlife Society (NEWS), Nature House, Gaudasahi, Angul, Odisha. <[email protected]>. * Corresponding Author. We describe a new species of the endemic frog genus Nasikabatrachus,from the eastern slopes of the Western Ghats, in India. The new species is morphologically, acoustically and genetically distinct from N. sahyadrensis. Computed tomography scans of both species revealed diagnostic osteological differences, particularly in the vertebral column. Male advertisement call analysis also showed the two species to be distinct. A phenological difference in breeding season exists between the new species (which breeds during the northeast monsoon season; October to December), and its sister species (which breeds during the southwest monsoon; May to August). The new species shows 6 % genetic divergence (K2P) at mitochondrial 16S rRNA (1330 bp) partial gene from its congener, indicating clear differentiation within Nasikabatra- chus. Speciation within this fossorial lineage is hypothesized to have been caused by phenological shift in breeding during different monsoon seasons—the northeast monsoon in the new species versus southwest monsoon in N. -

The Origins of Chordate Larvae Donald I Williamson* Marine Biology, University of Liverpool, Liverpool L69 7ZB, United Kingdom
lopmen ve ta e l B Williamson, Cell Dev Biol 2012, 1:1 D io & l l o l g DOI: 10.4172/2168-9296.1000101 e y C Cell & Developmental Biology ISSN: 2168-9296 Research Article Open Access The Origins of Chordate Larvae Donald I Williamson* Marine Biology, University of Liverpool, Liverpool L69 7ZB, United Kingdom Abstract The larval transfer hypothesis states that larvae originated as adults in other taxa and their genomes were transferred by hybridization. It contests the view that larvae and corresponding adults evolved from common ancestors. The present paper reviews the life histories of chordates, and it interprets them in terms of the larval transfer hypothesis. It is the first paper to apply the hypothesis to craniates. I claim that the larvae of tunicates were acquired from adult larvaceans, the larvae of lampreys from adult cephalochordates, the larvae of lungfishes from adult craniate tadpoles, and the larvae of ray-finned fishes from other ray-finned fishes in different families. The occurrence of larvae in some fishes and their absence in others is correlated with reproductive behavior. Adult amphibians evolved from adult fishes, but larval amphibians did not evolve from either adult or larval fishes. I submit that [1] early amphibians had no larvae and that several families of urodeles and one subfamily of anurans have retained direct development, [2] the tadpole larvae of anurans and urodeles were acquired separately from different Mesozoic adult tadpoles, and [3] the post-tadpole larvae of salamanders were acquired from adults of other urodeles. Reptiles, birds and mammals probably evolved from amphibians that never acquired larvae. -

Species Diversity and Conservation Status of Amphibians in Madre De Dios, Southern Peru
Herpetological Conservation and Biology 4(1):14-29 Submitted: 18 December 2007; Accepted: 4 August 2008 SPECIES DIVERSITY AND CONSERVATION STATUS OF AMPHIBIANS IN MADRE DE DIOS, SOUTHERN PERU 1,2 3 4,5 RUDOLF VON MAY , KAREN SIU-TING , JENNIFER M. JACOBS , MARGARITA MEDINA- 3 6 3,7 1 MÜLLER , GIUSEPPE GAGLIARDI , LILY O. RODRÍGUEZ , AND MAUREEN A. DONNELLY 1 Department of Biological Sciences, Florida International University, 11200 SW 8th Street, OE-167, Miami, Florida 33199, USA 2 Corresponding author, e-mail: [email protected] 3 Departamento de Herpetología, Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos, Avenida Arenales 1256, Lima 11, Perú 4 Department of Biology, San Francisco State University, 1600 Holloway Avenue, San Francisco, California 94132, USA 5 Department of Entomology, California Academy of Sciences, 55 Music Concourse Drive, San Francisco, California 94118, USA 6 Departamento de Herpetología, Museo de Zoología de la Universidad Nacional de la Amazonía Peruana, Pebas 5ta cuadra, Iquitos, Perú 7 Programa de Desarrollo Rural Sostenible, Cooperación Técnica Alemana – GTZ, Calle Diecisiete 355, Lima 27, Perú ABSTRACT.—This study focuses on amphibian species diversity in the lowland Amazonian rainforest of southern Peru, and on the importance of protected and non-protected areas for maintaining amphibian assemblages in this region. We compared species lists from nine sites in the Madre de Dios region, five of which are in nationally recognized protected areas and four are outside the country’s protected area system. Los Amigos, occurring outside the protected area system, is the most species-rich locality included in our comparison. -

Missouri's Toads and Frogs Booklet
TOADSMissouri’s andFROGS by Jeffrey T. Briggler and Tom R. Johnson, Herpetologists www.MissouriConservation.org © 1982, 2008 Missouri Conservation Commission Equal opportunity to participate in and benefit from programs of the Missouri Department of Conservation is available to all individuals without regard to their race, color, national origin, sex, age or disability. Questions should be directed to the Department of Conservation, P.O. Box 180, Jefferson City, MO 65102, (573) 751-4115 (voice) or 800-735-2966 (TTY), or to the U.S. Fish and Wildlife Service Division of Federal Assistance, 4401 N. Fairfax Drive, Mail Stop: MBSP-4020, Arlington, VA 22203. Cover photo: Eastern gray treefrog by Tom R. Johnson issouri toads and frogs are colorful, harmless, vocal and valuable. Our forests, prairies, rivers, swamps and marshes are Mhome to a multitude of toads and frogs, but few people know how many varieties we have, how to tell them apart, or much about their natural history. Studying these animals and sharing their stories with fellow Missourians is one of the most pleasurable and rewarding aspects of our work. Toads and frogs are amphibians—a class Like most of vertebrate animals that also includes amphibians, salamanders and the tropical caecilians, which are long, slender, wormlike and legless. frogs and Missouri has 26 species and subspecies (or toads have geographic races) of toads and frogs. Toads and frogs differ from salamanders by having an aquatic relatively short bodies and lacking tails at adulthood. Being an amphibian means that tadpole stage they live two lives: an aquatic larval or tadpole and a semi- stage and a semi-aquatic or terrestrial adult stage. -

Responses to Nonlethal Predators and the Roles of Competition and Resource Use
Virginia Commonwealth University VCU Scholars Compass Theses and Dissertations Graduate School 2011 SPECIES LEVEL DIFFERENCES IN THE ECOLOGY OF TWO NEOTROPICAL TADPOLE SPECIES: RESPONSES TO NONLETHAL PREDATORS AND THE ROLES OF COMPETITION AND RESOURCE USE Zacharia Costa Virginia Commonwealth University Follow this and additional works at: https://scholarscompass.vcu.edu/etd Part of the Biology Commons © The Author Downloaded from https://scholarscompass.vcu.edu/etd/2635 This Thesis is brought to you for free and open access by the Graduate School at VCU Scholars Compass. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of VCU Scholars Compass. For more information, please contact [email protected]. © Zacharia J. Costa All Rights Reserved SPECIES LEVEL DIFFERENCES IN THE ECOLOGY OF TWO NEOTROPICAL TADPOLE SPECIES: RESPONSES TO NONLETHAL PREDATORS AND THE ROLES OF COMPETITION AND RESOURCE USE A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIRMENTS FOR THE DEGREE OF MASTER OF SCIENCE AT VIRGINIA COMMONWEALTH UNIVERSITY by ZACHARIA J. COSTA Bachelor of Science, University of California, Davis 2007 Director DR. JAMES VONESH DEPARTMENT OF BIOLOGY Virginia Commonwealth University Richmond, Virginia December 13, 2011 Acknowledgments This would not have been possible if not for the support of my parents Kim and Marty Costa. They have always encouraged my pursuit of knowledge and love of the natural world, and are thus directly responsible for my career as a biologist. They have supported me in all of my endeavors often at their own expense, financially and emotionally, and for that I will forever be grateful. I also want to express my deep gratitude and thanks to my advisor Dr. -

Etar a Área De Distribuição Geográfica De Anfíbios Na Amazônia
Universidade Federal do Amapá Pró-Reitoria de Pesquisa e Pós-Graduação Programa de Pós-Graduação em Biodiversidade Tropical Mestrado e Doutorado UNIFAP / EMBRAPA-AP / IEPA / CI-Brasil YURI BRENO DA SILVA E SILVA COMO A EXPANSÃO DE HIDRELÉTRICAS, PERDA FLORESTAL E MUDANÇAS CLIMÁTICAS AMEAÇAM A ÁREA DE DISTRIBUIÇÃO DE ANFÍBIOS NA AMAZÔNIA BRASILEIRA MACAPÁ, AP 2017 YURI BRENO DA SILVA E SILVA COMO A EXPANSÃO DE HIDRE LÉTRICAS, PERDA FLORESTAL E MUDANÇAS CLIMÁTICAS AMEAÇAM A ÁREA DE DISTRIBUIÇÃO DE ANFÍBIOS NA AMAZÔNIA BRASILEIRA Dissertação apresentada ao Programa de Pós-Graduação em Biodiversidade Tropical (PPGBIO) da Universidade Federal do Amapá, como requisito parcial à obtenção do título de Mestre em Biodiversidade Tropical. Orientador: Dra. Fernanda Michalski Co-Orientador: Dr. Rafael Loyola MACAPÁ, AP 2017 YURI BRENO DA SILVA E SILVA COMO A EXPANSÃO DE HIDRELÉTRICAS, PERDA FLORESTAL E MUDANÇAS CLIMÁTICAS AMEAÇAM A ÁREA DE DISTRIBUIÇÃO DE ANFÍBIOS NA AMAZÔNIA BRASILEIRA _________________________________________ Dra. Fernanda Michalski Universidade Federal do Amapá (UNIFAP) _________________________________________ Dr. Rafael Loyola Universidade Federal de Goiás (UFG) ____________________________________________ Alexandro Cezar Florentino Universidade Federal do Amapá (UNIFAP) ____________________________________________ Admilson Moreira Torres Instituto de Pesquisas Científicas e Tecnológicas do Estado do Amapá (IEPA) Aprovada em de de , Macapá, AP, Brasil À minha família, meus amigos, meu amor e ao meu pequeno Sebastião. AGRADECIMENTOS Agradeço a CAPES pela conceção de uma bolsa durante os dois anos de mestrado, ao Programa de Pós-Graduação em Biodiversidade Tropical (PPGBio) pelo apoio logístico durante a pesquisa realizada. Obrigado aos professores do PPGBio por todo o conhecimento compartilhado. Agradeço aos Doutores, membros da banca avaliadora, pelas críticas e contribuições construtivas ao trabalho. -

Cop18 Doc. 62 (Rev
Original language: Spanish CoP18 Doc. 62 (Rev. 1) CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ____________________ Eighteenth meeting of the Conference of the Parties Colombo (Sri Lanka), 23 May – 3 June 2019 Species specific matters DRAFT DECISIONS ON THE CONSERVATION OF AMPHIBIANS (AMPHIBIA) 1. This document has been submitted by Costa Rica.* 2. Amphibians are the most threatened class of vertebrates worldwide. According to the IUCN Red List of Threatened Species, "of the 6260 amphibian species assessed, nearly one-third of species (32.4 %) are globally threatened or extinct, representing 2030 species". This is considerably higher than the comparable figure for birds (13 %) or mammals (22 %) (IUCN Red List 2016). The number of species classified as Critically Endangered, Endangered, or Vulnerable has increased throughout the world due to a number of threats, including habitat loss, trade, and disease. While loss of habitat and disease are considered to be the major threats to amphibian populations, harvesting for human use is a further pressure, sometimes posing the greatest threat (IUCN, 2016). 3. Amphibians occur throughout the world, with diversity nuclei in Central America, South America, East and Central Africa, East and South Asia, and Madagascar (Abraham et al. 2013; Jenkins et al. 2013; Pratihar et al. 2014; Pimm et al. 2014, among other authors). Amphibians live in a variety of terrestrial and freshwater ecosystems, ranging from tropical forests to deserts (Stuart et al. 2008). 4. Amphibians have been an important part of human culture for millennia and traditionally served as a source of food (e.g., Mohneke et al. -

Chromosome Analysis of Five Brazilian
c Indian Academy of Sciences RESEARCH ARTICLE Chromosome analysis of five Brazilian species of poison frogs (Anura: Dendrobatidae) PAULA CAMARGO RODRIGUES1, ODAIR AGUIAR2, FLÁVIA SERPIERI1, ALBERTINA PIMENTEL LIMA3, MASAO UETANEBARO4 and SHIRLEI MARIA RECCO-PIMENTEL1∗ 1Departamento de Anatomia, Biologia Celular e Fisiologia, Instituto de Biologia, Universidade Estadual de Campinas, 13083-863 Campinas, São Paulo, Brazil 2Departamento de Biociências, Universidade Federal de São Paulo, Campus Baixada Santista, 11060-001 Santos, São Paulo, Brazil 3Coordenadoria de Pesquisas em Ecologia, Instituto Nacional de Pesquisas do Amazonas, 69011-970 Manaus, Amazonas, Brazil 4Departamento de Biologia, Universidade Federal de Mato Grosso do Sul, 70070-900 Campo Grande, Mato Grosso do Sul, Brazil Abstract Dendrobatid frogs have undergone an extensive systematic reorganization based on recent molecular findings. The present work describes karyotypes of the Brazilian species Adelphobates castaneoticus, A. quinquevittatus, Ameerega picta, A. galactonotus and Dendrobates tinctorius which were compared to each other and with previously described related species. All karyotypes consisted of 2n = 18 chromosomes, except for A. picta which had 2n = 24. The karyotypes of the Adelphobates and D. tinctorius species were highly similar to each other and to the other 2n = 18 previously studied species, revealing conserved karyotypic characteristics in both genera. In recent phylogenetic studies, all Adelphobates species were grouped in a clade separated from the Dendrobates species. Thus, we hypothesized that their common karyotypic traits may have a distinct origin by chromosome rearrangements and mutations. In A. picta, with 2n = 24, chromosome features of pairs from 1 to 8 are shared with other previously karyotyped species within this genus. Hence, the A. -
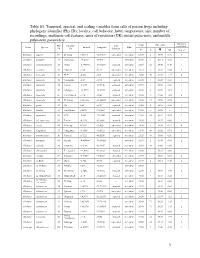
1 Table S1. Temporal, Spectral, and Scaling Variables from Calls Of
Table S1. Temporal, spectral, and scaling variables from calls of poison frogs including phylogeny identifier (Phy ID), locality, call behavior, habit, temperature, size, number of recordings, multinote call features, units of repetition (UR), initial pulse-note, and middle pulse-note parameters. Analyzed Phy Locality Call Temp SVL (mm) Genus Species Latitude Longitude Habit recordings ID ID Behavior °C N ! SD N of ♂ Allobates algorei 60 El Tama 7.65375 -72.19137 concealed terrestrial 23.50 8 18.90 0.70 3 Allobates brunneus 37 Guimaraes -15.2667 -55.5311 -- terrestrial 26.50 1 16.13 0.00 1 Allobates caeruleodactylus 48 Borba -4.398593 -59.60251 exposed terrestrial 25.60 12 15.50 0.40 1 Allobates crombiei 52 Altamira -3.65 -52.38 concealed terrestrial 24.10 2 18.10 0.04 2 Allobates femoralis 43 ECY -0.633 -76.5 concealed terrestrial 25.60 20 23.58 1.27 6 Allobates femoralis 46 Porongaba -8.67 -72.78 exposed terrestrial 25.00 1 25.38 0.00 1 Allobates femoralis 44 Leticia -4.2153 -69.9406 exposed terrestrial 25.50 1 20.90 0.00 1 Allobates femoralis 40 Albergue -12.8773 -71.3865 exposed terrestrial 26.00 6 21.98 2.18 1 Allobates femoralis 41 CAmazonico -12.6 -70.08 exposed terrestrial 26.00 12 22.43 1.06 4 Allobates femoralis 45 El Palmar 8.333333 -61.66667 concealed terrestrial 24.00 27 25.50 0.76 1 Allobates granti 49 FG 3.62 -53.17 exposed terrestrial 24.60 8 16.15 0.55 1 Allobates humilis 59 San Ramon 8.8678 -70.4861 concealed terrestrial 19.50 -- 21.80 -- 1 Allobates insperatus 54 ECY -0.633 -76.4005 exposed terrestrial 24.60 18 16.64 0.93 7 Allobates aff. -

Taxonomic Checklist of Amphibian Species Listed in the CITES
CoP17 Doc. 81.1 Annex 5 (English only / Únicamente en inglés / Seulement en anglais) Taxonomic Checklist of Amphibian Species listed in the CITES Appendices and the Annexes of EC Regulation 338/97 Species information extracted from FROST, D. R. (2015) "Amphibian Species of the World, an online Reference" V. 6.0 (as of May 2015) Copyright © 1998-2015, Darrel Frost and TheAmericanMuseum of Natural History. All Rights Reserved. Additional comments included by the Nomenclature Specialist of the CITES Animals Committee (indicated by "NC comment") Reproduction for commercial purposes prohibited. CoP17 Doc. 81.1 Annex 5 - p. 1 Amphibian Species covered by this Checklist listed by listed by CITES EC- as well as Family Species Regulation EC 338/97 Regulation only 338/97 ANURA Aromobatidae Allobates femoralis X Aromobatidae Allobates hodli X Aromobatidae Allobates myersi X Aromobatidae Allobates zaparo X Aromobatidae Anomaloglossus rufulus X Bufonidae Altiphrynoides malcolmi X Bufonidae Altiphrynoides osgoodi X Bufonidae Amietophrynus channingi X Bufonidae Amietophrynus superciliaris X Bufonidae Atelopus zeteki X Bufonidae Incilius periglenes X Bufonidae Nectophrynoides asperginis X Bufonidae Nectophrynoides cryptus X Bufonidae Nectophrynoides frontierei X Bufonidae Nectophrynoides laevis X Bufonidae Nectophrynoides laticeps X Bufonidae Nectophrynoides minutus X Bufonidae Nectophrynoides paulae X Bufonidae Nectophrynoides poyntoni X Bufonidae Nectophrynoides pseudotornieri X Bufonidae Nectophrynoides tornieri X Bufonidae Nectophrynoides vestergaardi