Determination of Pyrrolizidine Alkaloids Levels in Symphytum Asperum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FDA OTC Reviews Summary of Back Issues
Number 23 The Journal of the AMERICAN BOTANI CAL COUNCIL and the HERB RESEARCH FOUNDATION Chinese Medicinals -A Comprehensive Review of Chinese Materia Medica Legal and Regulatory- FDA OTC Reviews Summary of Back Issues Ongoing Market Report, Research Reviews (glimpses of studies published in over a dozen scientific and technical journals), Access, Book Reviews, Calendar, Legal and Regulatory, Herb Blurbs and Potpourri columns. #1 -Summer 83 (4 pp.) Eucalyptus Repels Reas, Stones Koalas; FDA OTC tiveness; Fungal Studies; More Polysaccharides; Recent Research on Ginseng; Heart Panel Reviews Menstrual & Aphrodisiac Herbs; Tabasco Toxicity?; Garlic Odor Peppers; Yew Continues to Amaze; Licorice O.D. Prevention; Ginseng in Perspec Repels Deer; and more. tive; Poisonous Plants Update; Medicinal Plant Conservation Project; 1989 Oberly #2- Fall/Winter 83-84 (8 pp.) Appeals Court Overrules FDA on Food Safety; Award Nominations; Trends in Self-Care Conference; License Plates to Fund Native FDA Magazine Pans Herbs; Beware of Bay Leaves; Tiny Tree: Cancer Cure?; Plant Manual; and more. Comfrey Tea Recall; plus. #17-Summer 88. (24 pp.) Sarsaparilla, A Literature Review by Christopher #3-Spring 84 (8 pp.) Celestial Sells to Kraft; Rowers and Dinosaurs Demise?; Hobbs; Hops May Help Metabolize Toxins; Herbal Roach Killer; Epazote Getting Citrus Peels for Kitty Litter; Saffron; Antibacterial Sassafras; WHO Studies Anti· More Popular, Aloe Market Levels Off; Herbal Tick Repellent?; Chinese Herb fertility Plants; Chinese Herbal Drugs; Feverfew Migraines; -

Diplomová Práce
Jihočeská univerzita v Českých Budějovicích Zemědělská fakulta Studijní program: N4101 Zemědělské inženýrství Studijní obor: Agroekologie Katedra: Rostlinné výroby a agroekologie Vedoucí katedry: prof. Ing. Vladislav Čurn, Ph.D. DIPLOMOVÁ PRÁCE Biologie, semenářské vlastnosti a zakládání porostů kostivalu lékařského (Symphytum officinale L.) Vedoucí diplomové práce: Ing. Milan Kobes, Ph.D. Autor diplomové práce: Bc. Jaroslava Kovářová České Budějovice, duben 2016 Čestné prohlášení Prohlašuji, že svou diplomovou práci s názvem „Biologie, semenářské vlastnosti a zakládání porostů kostivalu lékařského (Symphytum officinale L.)“ jsem vypracovala samostatně pod vedením vedoucí diplomové práce Ing. Milana Kobese, Ph.D a s použitím odborné literatury a dalších informačních zdrojů, které jsou v práci citovány a zároveň uvedeny v seznamu literatury na konci této práce. Jako autorka uvedené diplomové práce dále prohlašuji, že jsem v souvislosti s jejím vytvořením neporušila autorská práva třetích osob. Dále prohlašuji, že v souladu s § 47b zákona č. 111/1998 Sb. v platném znění souhlasím se zveřejněním své diplomové práce, a to v nezkrácené podobě (v úpravě vzniklé vypuštěním vyznačených částí archivovaných Zemědělskou fakultou JU) elektronickou cestou ve veřejně přístupné části databáze STAG provozované Jihočeskou univerzitou v Českých Budějovicích na jejích internetových stránkách. V Českých Budějovicích dne.......... _____________________________ Poděkování Velké poděkování patří mému vedoucímu práce Ing. Milanu Kobesovi, Ph.D. za odborné rady, připomínky, za vedení a odborné konzultace, které mi poskytl během zpracování této diplomové práce. Děkuji celé své rodině za podporu. ABSTRAKT Diplomová práce s názvem ,,Biologie, semenářské vlastnosti a zakládání porostů kostivalu lékařského (Symphytum officinale L.)“ se zabývala hodnocením způsobů rozmnožování kostivalu a hodnocením vlivu ekologických podmínek na růst kostivalu. Dále testy klíčivosti a jejich statistickým hodnocením při respektování specifik práce se semeny. -
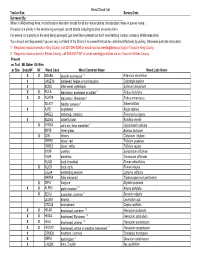
Weed Check List Survey Date
Weed Check List Trail or Site: Survey Date: Surveyed By: When in Wildnerness Area, record location and other details for all non-native plants. Include plant name in survey notes. If a weed is a priority in the area being surveyed, record details including location on survey form. For weeds not a priority in the area being surveyed, just mark them present but don't need lat/long location unless in Wilderness Area. You can pull and bag weeds if you are very confident of the ID and it is a weed that can be controlled effectively by pulling. Otherwise just note its location. 1 - Regulated noxious weeds in King County, call 206-296-0290 or email [email protected] if found in King County 2 - Regulated noxious weed in Kittitas County, call 509-962-7007 or email [email protected] if found in Kittitas County Present on Trail Mt. Baker Ok Wen or Site Snoq NF NF Weed Code Weed Common Name Weed Latin Name XXARAB3 absinth wormwood 1,2 Artemisia absinthium X CASE13 bindweed, hedge or morning glory Calystegia sepium X SODU bittersweet nightshade Solanum dulcamara XXRULA blackberry, evergreen or cutleaf 2 Rubus laciniatus XXRUAR9 blackberry, Himalayan 2 Rubus armeniacus SILA21 bladder campion 2 Silene latifolia X AJRE bugleweed Ajuga reptans RARE3 buttercup, creeping Ranunculus repens X BUDA2 butterfly bush Buddleia davidii X HYRA3 cat’s ear, false dandelion 2 Hypochaeris radicata BRTE cheat grass Bromus tectorum X CIIN chicory Cichorium intybus TRPR2 clover, red Trifolium pratense TRRE3 clover, white Trifolium repens SYOF comfrey Symphytum officinale TAOF dandelion Taraxacum officinale RUOB dock, broadleaf Rumex obtusifolius X RUCR dock, curly Rumex crispus LALA4 everlasting peavine Lathyrus latifolius MAPE2 false mayweed Tripleurospermum perforatum X DIPU foxglove Digitalis purpurea XXALPE4 garlic mustard 1,2 Alliaria petiolata X SEVU groundsel, common 2 Senecio vulgaris LEONT hawkbit Leontodon spp. -

Sterol Addition During Pollen Collection by Bees
Sterol addition during pollen collection by bees: another possible strategy to balance nutrient deficiencies? Maryse Vanderplanck, Pierre-Laurent Zerck, Georges Lognay, Denis Michez To cite this version: Maryse Vanderplanck, Pierre-Laurent Zerck, Georges Lognay, Denis Michez. Sterol addition during pollen collection by bees: another possible strategy to balance nutrient deficiencies?. Apidologie, 2020, 51 (5), pp.826-843. 10.1007/s13592-020-00764-3. hal-02784696 HAL Id: hal-02784696 https://hal.archives-ouvertes.fr/hal-02784696 Submitted on 3 May 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Apidologie (2020) 51:826–843 Original article * INRAE, DIB and Springer-Verlag France SAS, part of Springer Nature, 2020 DOI: 10.1007/s13592-020-00764-3 Sterol addition during pollen collection by bees: another possible strategy to balance nutrient deficiencies? 1,2 1 3 1 Maryse VANDERPLANCK , Pierre-Laurent ZERCK , Georges LOGNAY , Denis MICHEZ 1Laboratory of Zoology, Research Institute for Biosciences, University of Mons, 20 Place du Parc, 7000, Mons, Belgium 2CNRS, UMR 8198 - Evo-Eco-Paleo, Univ. Lille, F-59000, Lille, France 3Analytical Chemistry, Agro Bio Chem Department, Gembloux Agro-Bio Tech University of Liège, 2 Passage des Déportés, 5030, Gembloux, Belgium Received 10 July 2019 – Revised2March2020– Accepted 30 March 2020 Abstract – Sterols are essential nutrients for bees which are thought to obtain them exclusively from pollen. -

Vascular Plants of Humboldt Bay's Dunes and Wetlands Published by U.S
Vascular Plants of Humboldt Bay's Dunes and Wetlands Published by U.S. Fish and Wildlife Service G. Leppig and A. Pickart and California Department of Fish Game Release 4.0 June 2014* www.fws.gov/refuge/humboldt_bay/ Habitat- Habitat - Occurs on Species Status Occurs within Synonyms Common name specific broad Lanphere- Jepson Manual (2012) (see codes at end) refuge (see codes at end) (see codes at end) Ma-le'l Units UD PW EW Adoxaceae Sambucus racemosa L. red elderberry RF, CDF, FS X X N X X Aizoaceae Carpobrotus chilensis (Molina) sea fig DM X E X X N.E. Br. Carpobrotus edulis ( L.) N.E. Br. Iceplant DM X E, I X Alismataceae lanceleaf water Alisma lanceolatum With. FM X E plantain northern water Alisma triviale Pursh FM X N plantain Alliaceae three-cornered Allium triquetrum L. FS, FM, DM X X E leek Allium unifolium Kellogg one-leaf onion CDF X N X X Amaryllidaceae Amaryllis belladonna L. belladonna lily DS, AW X X E Narcissus pseudonarcissus L. daffodil AW, DS, SW X X E X Anacardiaceae Toxicodendron diversilobum Torrey poison oak CDF, RF X X N X X & A. Gray (E. Greene) Apiaceae Angelica lucida L. seacoast angelica BM X X N, C X X Anthriscus caucalis M. Bieb bur chevril DM X E Cicuta douglasii (DC.) J. Coulter & western water FM X N Rose hemlock Conium maculatum L. poison hemlock RF, AW X I X Daucus carota L. Queen Anne's lace AW, DM X X I X American wild Daucus pusillus Michaux DM, SW X X N X X carrot Foeniculum vulgare Miller sweet fennel AW, FM, SW X X I X Glehnia littoralis (A. -

Shoot Structure of Symphytum Officinale L. (Boraginaceae) in Relation to the Nature of Its Axially Shifted Lateral Branches
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Wulfenia Jahr/Year: 2011 Band/Volume: 18 Autor(en)/Author(s): Kotelnikova Ksenia V., Tretjakova Daria J., Nuraliev Maxim S. Artikel/Article: Shoot structure of Symphytum officinale L. (Boraginaceae) in relation to the nature of its axially shifted lateral branches. 63-79 © Landesmuseum für Kärnten; download www.landesmuseum.ktn.gv.at/wulfenia; www.biologiezentrum.at Wulfenia 18 (2011): 63 –79 Mitteilungen des Kärntner Botanikzentrums Klagenfurt Shoot structure of Symphytum offi cinale L. (Boraginaceae) in relation to the nature of its axially shifted lateral branches Ksenia V. Kotelnikova, Daria J. Tretjakova & Maxim S. Nuraliev Summary: Shoot structure of Symphytum offi cinale was studied in terms of its architecture, vascular anatomy and morphogenesis. The main stem and lower stems of secondary order typically bear axially shifted lateral branches (along with axillary ones). This results in development of internode-like stem section between the lateral branch and its subtending leaf. Each shifted branch possesses two stem leaves (prophylls) and a terminal infl orescence. All lateral buds are initiated in the leaf axils. At later developmental stages some of them become shifted due to the intercalary growth of the stem between them and their subtending leaves. The intercalary stem portion possesses internodal stele structure, and the stele of the lateral stem branches from the main stele almost at the level of lateral stem insertion. This phenomenon can be treated as concaulescence (i.e. congenital fusion), though no strict evidences for phylogenetic fusion were found. Stems of Symphytum offi cinale also bear green wings of leafy nature. -

Boraginaceae), and the Phylogeny of Boraginoideae
!" #$ % " "& '()*"'+ (,-./01 ** -)2'/)*) %*()'-) %%*(* 3443 Dissertation for the Degree of Doctor of Philosophy in Systematic Botany presented at Uppsala University in 2002 Abstract Långström, E. 2002. Systematics of Echiochilon and Ogastemma (Boraginaceae), and the phylogeny of Boraginoideae. Acta Univ. Ups. Comprehensive Summaries og Uppsala Disserta- tions from the Faculty of Science and Technology 693. 34 pp. Uppsala. ISBN 91-554-5257-4. Echiochilon, Ogastemma and Sericostoma are revised resulting in the recognition of 15 spe- cies of Echiochilon and one Ogastemma species. Several species are placed in synonymy and three new species are described, E. baricum, E. callianthum and E. cyananthum. The single species of Sericostoma is shown to be nested within Echiochilon. The plastid atpB gene was sequenced for Echiochilon and Ogastemma from the Old World and Antiphytum from the New World, plus for a selection of 33 other Boraginaceae taxa. They were analysed together with selected outgroup taxa to give a framework of the tribes of Boraginoi- deae. The analysis gave support for establishing the new tribe Echiochileae for Antiphytum, Echio- chilon and Ogastemma, and for merging the traditionally accepted tribe Eritrichieae with Cyno- glosseae. The ITS region was sequenced for all but one species of Echiochilon and for representa- tives of Antiphytum and Ogastemma. Phylogenetic analysis of Echiochilon revealed that the strongly zygomorphic-flowered species form a paraphyletic group. The morphological data gave results fairly congruent with the ITS phylogeny. Biogeographic interpretations of the ITS and atpB phylogenies indicated a trans-Atlantic dispersal of Antiphytum as the most plausible explanation to the Old/New World disjunction. Analyses using DIVA (Dispersal Vicariance Analysis) of the distributions of the Echiochilon spe- cies indicated an ancestor to Echiochilon with a wide distribution over northern Africa and Arabia to India. -

Phylogeny of Eudicots (Or Tricolpates) Eudicots (Or Tricolpates) “Basal Eudicots”
Phylogeny of Eudicots (or Tricolpates) Eudicots (or Tricolpates) “Basal eudicots” Asterids Buxales Rosids Caryophyllales RanunculalesProteales After Jansen et al., 2007, Proc. Natl. Acad. Sci. USA 104: 19369-19374 Phylogeny of Asterids Asterids Lamiids Ericales Campanulids Cornales Cornales Ericales Campanulids: Lamiids: Aquifoliales Garryales Apiales Gentianales Dipsacales Lamiales Asterales Solanales After APG, 2003; Judd and Olmstead, 2004, and Soltis et al., 2005 Synapomorphies for Asterids and Core Asterids Synapomorphies for Asterids: Iridoid compounds; Unitegmic ovules; www.scielo.br/img/fbpe/jbchs/v12n2/a04fig01.gif Tenuinucellate ovules. Synapomorphies for Core Asterids (Lamiids + Campanulids): Gamopetalous corollas; A single whorl of stamens that alternate with the petal lobes; Epipetalous stamens; 2 fused carpels. Ericaceae (Rhododendron family) http://georgian.wunderground.com/data/wximagenew/f/Feather3/5431.jpg Rhododendron macrophyllum (Pacific Rhododendron, WA State Flower) Ericaceae (Rhododendron family) Textbook DVD WSJ Textbook DVD KRR & DLN Vaccinium corymbosum; The genus Vaccinium contains about 450 species, including blueberry, cranberry, huckleberry, etc.. Ericaceae (Rhododendron family) Photo: Yaowu Yuan Photo: Yaowu Yuan Gautheria shallon; Salal Cassiope tetragona http://www.nestlerode.org/ Cremation_Urn/urn_finished.jpg Photo: Yaowu Yuan Note the more or less pendulous Menziesia ferruginea flowers and the urn-shaped corolla. Ericaceae (Rhododendron family) Textbook DVD KRR & DLN Textbook DVD KMN Pieris japonica Vaccinium arboreum Note that the anthers often have spur- or awn-like appendages. Ericaceae (Rhododendron family) Photo: Yaowu Yuan Menziesia ferruginea Note that anther opens by 2 terminal pores –– poricidal dehiscence. Ericaceae (Rhododendron family) Textbook DVD KRR & DLN Kalmia latifolia Note that although corolla can be more spread and not urn-shaped, anther usually opens by terminal pores. -

Distributions of Vascular Plants in the Czech Republic. Part 3
Preslia 88: 459–544, 2016 459 Distributions of vascular plants in the Czech Republic. Part 3 Rozšíření cévnatých rostlin v České republice. Část 3 Zdeněk K a p l a n1,JiříDanihelka1, 2,MartinLepší3, 4,PetrLepší5, Libor E k r t4, Jindřich C h r t e k Jr.1,JiříKocián6,JanPrančl1, 7, LucieKobrlová8,MichalHroneš8 & Václav Š u l c1 1Institute of Botany, The Czech Academy of Sciences, Zámek 1, CZ-252 43 Průhonice, Czech Republic, e-mail: [email protected], [email protected], [email protected], [email protected]; 2Department of Botany and Zoology, Masaryk University, Kotlářská 2, CZ-611 37 Brno, Czech Republic, e-mail: [email protected]; 3South Bohemian Museum in České Budějovice, Dukelská 1, CZ-370 51 České Budějovice, Czech Republic, e-mail: [email protected]; 4Department of Botany, Faculty of Science, University of South Bohemia, Branišovská 1760, CZ-370 05 České Budějovice, Czech Republic, e-mail: [email protected]; 5Nature Conservation Agency of the Czech Republic, Administration of the Blanský les Protected Landscape Area, Vyšný 59, CZ-381 01 Český Krumlov, Czech Republic, e-mail: [email protected]; 6Nature Conservation Agency of the Czech Republic, Kaplanova 1, CZ-148 00 Prague, Czech Republic, e-mail: [email protected]; 7Department of Botany, Faculty of Science, Charles University, Benátská 2, CZ-128 01 Prague, Czech Republic; 8Department of Botany, Faculty of Science, Palacký University, Šlechtitelů 27, CZ-783 71 Olomouc, e-mail: lucka.kobrlova @seznam.cz, [email protected] Kaplan Z., Danihelka J., Lepší M., Lepší P., Ekrt L., Chrtek J. -

Boraginaceae) Taxa from Turkey
Bangladesh J. Bot. 36(2): 93-103, 2007 (December) MICROMORPHOLOGY AND ANATOMY OF THREE SYMPHYTUM (BORAGINACEAE) TAXA FROM TURKEY OZNUR ERGEN AKCIN AND HILAL BAKI Department of Biology, Sciences & Arts Faculty, Ordu University, Ordu, Turkey E-mail: [email protected] Key words: Micromorphology, Anatomy, Symphytum Abstract Symphytum asperum Lepechin, S. ibericum Steven and S. sylvaticum Boiss. were examined morphologically, micromorphologically and anatomically. Scanning electron microscopy was used to examine leaf surface and trichomes of these species. These species had bifacial and hypostomatous leaf types. Epidermal cells of leaves were usually polygonal or irregular in form. The pattern of anticlinical cells may vary in different species and between the upper and lower epidermis of the same species. Stomata are anisocytic and anomocytic in three species. Stomata index is 27.5 for S. sylvaticum, 24.65 for S. ibericum and 21.86 for S. asperum glandular trichomes are capitate in forms and more dense on the lower epidermis than upper epidermis. Eglandular trichomes are simple, short or long, unicellular or multicellular and thin or thick. Introduction Symphytum L. (Boraginaceae) is represented by about 23 taxa in Turkey (Wickens 1978, Yildirimli 2000). Symphytum species are common garden plants and have been used as a source of herbal medicines for >2000 years. Leaf and root of Symphytum species are used by lay public, herbalists and physicians for treatment of broken bones, tendon damage, ulcerations in the gastrointestinal tract, and lung congestion. These species are also rich in many crucial nutrients, such as protein, antioxidant, vitamins and vitamin B12, and are common component in the diet of certain ethnic groups (Hills 1976, Rode 2002). -
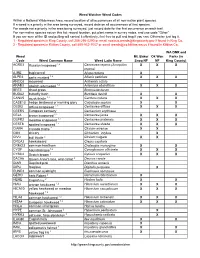
Trail Weed Watcher Weed Check List
Weed Watcher Weed Codes Within a National Wilderness Area, record location of all occurrences of all non-native plant species. If a weed is a priority in the area being surveyed, record data on all occurrences of that species. For weeds not a priority in the area being surveyed, just record data for the first occurrence on each trail. For non-native species not on this list: record location, put plant name in survey notes, and use code "Other" If you are sure of the ID and pulling will control it effectively, feel free to pull and bag if you can. Otherwise just log it. 1 - Regulated species in King County, call 206-296-0290 or email [email protected] if found in King Co. 2 - Regulated species in Kittitas County, call 509-962-7007 or email [email protected] if found in Kittitas Co. WA DNR and Weed Mt. Baker Ok Wen Parks (in Code Weed Common Name Weed Latin Name Snoq NF NF King County) ACRE3 Russian knapweed 1,2 Centaurea repens (Acroptilon XX X repens) AJRE bugleweed Ajuga reptans X ALPE4 garlic mustard 1,2 Alliaria petiolata XX X ANCO2 mayweed Anthemis cotula ARAB3 absinth wormwood 1,2 Artemisia absinthium XX X BRTE cheat grass Bromus tectorum BUDA2 butterfly bush Buddleia davidii X X CANU5 musk thistle 1,2 Carduus nutans XX X CASE13 hedge bindweed or morning glory Calystegia sepium X X CEDI3 diffuse knapweed 1,2 Centaurea diffusa XX X CEER5 European centaury Centaurium erythraea CEJA brown knapweed 1,2 Centaurea jacea XX X CEPR2 meadow knapweed 1,2 Centaurea pratensis XX X CEST8 spotted knapweed 1,2 Centaurea stoebe -
![Comfrey [72698-57-8] and One of Its Constituent Alkaloids](https://docslib.b-cdn.net/cover/3380/comfrey-72698-57-8-and-one-of-its-constituent-alkaloids-3123380.webp)
Comfrey [72698-57-8] and One of Its Constituent Alkaloids
Comfrey [72698-57-8] and One of Its Constituent Alkaloids Symphytine [22571-95-5] Review of Toxicological Literature Prepared for Errol Zeiger, Ph.D. National Institute of Environmental Health Sciences P.O. Box 12233 Research Triangle Park, North Carolina 27709 Contract No. N01-ES-65402 Submitted by Raymond Tice, Ph.D. Integrated Laboratory Systems P.O. Box 13501 Research Triangle Park, North Carolina 27709 October 1997 EXECUTIVE SUMMARY The nomination of comfrey and symphytine by the NIEHS for testing is based on the potential for chronic human exposure and the limited amount of carcinogenicity data. Comfrey is a member of the plant family Boraginaceae. The major constituent of the comfrey plant is mucilage; other constituents include allantoin, polyphenols, amino acids, phytosterols, triterpenoids, saccharides, and pyrrolizidine alkaloids. The major unsaturated pyrrolizidine alkaloids in comfrey are lycopsamine, intermedine, 7-acetyllycopsamine, 7-acetylintermedine, and symphytine. One kilogram of comfrey contains 1.7-2.5 grams of symphytine. Comfrey is commercially available as a root or leaf, and in capsule form. In 1993, it was reported that some suppliers had removed comfrey from the U.S. market in response to adverse toxicological findings. Celestial Seasonings, a major producer of herbal teas, removed comfrey from its product line in 1981. Symphytine can be extracted from the dried roots of comfrey using methanol. Allantoin is removed from the resulting crude extract and the residue can then be purified by reduction of the N- oxide with zinc dust and hydrochloric acid, followed by column chromatography. Production and import volumes were not available for comfrey; symphytine is not produced commercially.