Towards a Natural Classification of Sapotaceae Subfamily Chrysophylloideae in the Neotropics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Renata Gabriela Vila Nova De Lima Filogenia E Distribuição
RENATA GABRIELA VILA NOVA DE LIMA FILOGENIA E DISTRIBUIÇÃO GEOGRÁFICA DE CHRYSOPHYLLUM L. COM ÊNFASE NA SEÇÃO VILLOCUSPIS A. DC. (SAPOTACEAE) RECIFE 2019 RENATA GABRIELA VILA NOVA DE LIMA FILOGENIA E DISTRIBUIÇÃO GEOGRÁFICA DE CHRYSOPHYLLUM L. COM ÊNFASE NA SEÇÃO VILLOCUSPIS A. DC. (SAPOTACEAE) Dissertação apresentada ao Programa de Pós-graduação em Botânica da Universidade Federal Rural de Pernambuco (UFRPE), como requisito para a obtenção do título de Mestre em Botânica. Orientadora: Carmen Silvia Zickel Coorientador: André Olmos Simões Coorientadora: Liliane Ferreira Lima RECIFE 2019 Dados Internacionais de Catalogação na Publicação (CIP) Sistema Integrado de Bibliotecas da UFRPE Biblioteca Central, Recife-PE, Brasil L732f Lima, Renata Gabriela Vila Nova de Filogenia e distribuição geográfica de Chrysophyllum L. com ênfase na seção Villocuspis A. DC. (Sapotaceae) / Renata Gabriela Vila Nova de Lima. – 2019. 98 f. : il. Orientadora: Carmen Silvia Zickel. Coorientadores: André Olmos Simões e Liliane Ferreira Lima. Dissertação (Mestrado) – Universidade Federal Rural de Pernambuco, Programa de Pós-Graduação em Botânica, Recife, BR-PE, 2019. Inclui referências e anexo(s). 1. Mata Atlântica 2. Filogenia 3. Plantas florestais 4. Sapotaceae I. Zickel, Carmen Silvia, orient. II. Simões, André Olmos, coorient. III. Lima, Liliane Ferreira, coorient. IV. Título CDD 581 ii RENATA GABRIELA VILA NOVA DE LIMA Filogenia e distribuição geográfica de Chrysophyllum L. com ênfase na seção Villocuspis A. DC. (Sapotaceae Juss.) Dissertação apresentada e -

Amazon Alive!
Amazon Alive! A decade of discovery 1999-2009 The Amazon is the planet’s largest rainforest and river basin. It supports countless thousands of species, as well as 30 million people. © Brent Stirton / Getty Images / WWF-UK © Brent Stirton / Getty Images The Amazon is the largest rainforest on Earth. It’s famed for its unrivalled biological diversity, with wildlife that includes jaguars, river dolphins, manatees, giant otters, capybaras, harpy eagles, anacondas and piranhas. The many unique habitats in this globally significant region conceal a wealth of hidden species, which scientists continue to discover at an incredible rate. Between 1999 and 2009, at least 1,200 new species of plants and vertebrates have been discovered in the Amazon biome (see page 6 for a map showing the extent of the region that this spans). The new species include 637 plants, 257 fish, 216 amphibians, 55 reptiles, 16 birds and 39 mammals. In addition, thousands of new invertebrate species have been uncovered. Owing to the sheer number of the latter, these are not covered in detail by this report. This report has tried to be comprehensive in its listing of new plants and vertebrates described from the Amazon biome in the last decade. But for the largest groups of life on Earth, such as invertebrates, such lists do not exist – so the number of new species presented here is no doubt an underestimate. Cover image: Ranitomeya benedicta, new poison frog species © Evan Twomey amazon alive! i a decade of discovery 1999-2009 1 Ahmed Djoghlaf, Executive Secretary, Foreword Convention on Biological Diversity The vital importance of the Amazon rainforest is very basic work on the natural history of the well known. -

Pdf (Last Access on 14/02/2018)
Biota Neotropica 18(4): e20180590, 2018 www.scielo.br/bn ISSN 1676-0611 (online edition) Inventory Floristic and structure of the arboreal community of an Ombrophilous Dense Forest at 800 m above sea level, in Ubatuba/SP, Brazil Ana Cláudia Oliveira de Souza1* , Luís Benacci2 & Carlos Alfredo Joly3 1Universidade Estadual Paulista Júlio de Mesquita Filho, Departamento de Botânica, Campus de Rio Claro, Av. 24 A, 1515, 13506-900, Rio Claro, SP, Brasil 2Instituto Agronômico, 13020-902, Campinas, SP, Brasil 3Universidade de Campinas, Instituto de Biologia, Campinas, SP, Brasil *Corresponding author: Ana Cláudia Oliveira de Souza, e-mail: [email protected] SOUZA, A. C. O., BENACCI, L., JOLY, C. A. Floristic and structure of the arboreal community of an Ombrophilous Dense Forest at 800 m above sea level, in Ubatuba/SP, Brazil. Biota Neotropica. 18(4): e20180590. http://dx.doi.org/10.1590/1676-0611-BN-2018-0590 Abstract: Undoubtedly, the publication of floristic lists and phytosociological studies are important tools for metadata generation, quantification and characterization of the megadiversity of Brazilian forests. In this sense, this work had the objective of describing the composition and the structure of the tree community of one hectare of Dense Atlantic Rainforest, at an altitude of 800 m. All individuals, including trees, palm trees, arborescent ferns and dead and standing stems, with a diameter at breast height (DBH) of ≥ 4.8 cm were sampled. After the identification of the botanical material, we proceeded to calculate the usual phytosociological parameters, besides the Shannon diversity index (H’) and Pielou equability index (J). A total of 1.791 individuals were sampled, of which 1.729 were alive, belonging to 185 species, 100 genera and 46 families. -

Interacciones Mutualistas. X. Antropocoria: Florestas Neotropicales Publicacions Del CRBA
Interacciones mutualistas. X. Antropocoria: florestas neotropicales Publicacions del CRBA Interacciones mutualistas entre animales y plantas X. Antropocoria: florestas neotropicales Juan Carlos Guix Coordinador del Proyecto Neopangea e-mail: [email protected] 1 © Centre de Recursos de Biodiversitat Animal, Facultat de Biologia, Universitat de Barcelona. 2021. Maig, 2021 Publicat per: Centre de Recursos de Biodiversitat Animal Facultat de Biologia Universitat de Barcelona Avinguda Diagonal 643 08028 Barcelona Spain [email protected] www.ub.edu/crba Guix, J.C. 2021. Interacciones mutualistas entre animales y plantas. X. Antropocoria: florestas neotropicales. Publicacions del Centre de Recursos de Biodiversitat Animal. Universitat de Barcelona, Volum 16, 107 pp. Portada: Las pluvisilvas situadas en las laderas orientales de los Andes se encuentran entre los bosques tropicales más diversos del mundo. Provincia de Napo, Ecuador. Foto: Juan Carlos Guix. 2 Interacciones mutualistas. X. Antropocoria: florestas neotropicales Publicacions del CRBA Interacciones mutualistas entre animales y plantas X. Antropocoria: florestas neotropicales Juan Carlos Guix Con frecuencia se asocia la floresta amazónica u otros tipos de selvas tropicales con la noción de una “naturaleza virgen”. Sin embargo, las florestas tropicales y subtropicales del Neotrópico están habitadas desde hace más de 14.000 años por pueblos indígenas, y estos a su vez han producido cambios apreciables en las comunidades de plantas de estos ecosistemas. La diseminación de semillas grandes en florestas neotropicales Los humanos y las redes de interacciones animal-planta De un modo general, las interacciones entre los humanos modernos (Homo sapiens) y los sistemas de diseminación de semillas han sido expresadas en forma de impactos antropogénicos relacionados con la fragmentación de hábitats, la sobrecaza y la extinción, a escala local o regional, de frugívoros de medio y gran porte (cf. -

Tbiseries3.Pdf
b_[^LZE[aâ aL_QLaâ 5â bxâb¶¬²x§o¬Àâ ax¶xÀâ ²¶xÀx§ÅÀâÅxâ¶xÀÊÅÀâ¬|âÀÅÊuxÀâ j§uâ ¶xÀxj¶qâ jqÅÎÅxÀâ¶xjÅxuâŬâÅxâ q¬§Àx¶Îjà Ŭ§âj§uâÑÀxâÊÃÛjŬ§â¬|â¶xÀÅâj§uÀâ § âÅxâ Ê¢uâŶ¬²qÀ#âbxâ Àx¶xÀâq¬§Å§ÊxÀâj§uâ§Åx¶jÅxÀâÅxâ }®¶¢x¶âb¶¬²x§p¬Àâaqx§Åqâj§uâbxq§qjâax¶xÀ#âbxâÀÅÊuxÀâ²ÊoÀxuâ§âÅÀâÀx¶xÀâjÎxâoxx§âqj¶à ¶xuâ ¬ÊÅâÑŧâ Åxâ §Åx¶§jŬ§j âb¶¬²x§o¬ÀⲶ¬¶j¢¢x#â[qqjÀ¬§jÕ âÅÀâÀx¶xÀâ¢jÕⲶxÀx§ÅâÅxâ ¶xÀÊÅÀâ¬|â¬Åx¶âÀÅÊuxÀâÒqâq¬§Å¶oÊÅxâŬâÅxâ¬oxqÅÎxÀâ¬|âÅxâb¶¬²x§o¬ÀⲶ¬¶j¢¢x+â GQ^KDbDâ T\YQZTVQRULâ EQEVQ[bPLLTâKLYâPDDNâ aÅxxxâPj§ÀâÅz¶â ^jÅÅx¶§Àâ §â Ŷ¬´qjâ ¶j§â}®¶xÀÅâ§â NÊÕj§jâ5ĺPj§Àâ Åx¶âaÅxxx#â fjx§§x§?âbxâb¶¬²x§o¬ÀâM¬Ê§ujÞ Å¬§#â Qâčĺ1â  c¶¬²x§o¬ÀâÀx¶xÀâ bxÀÀâ_ÀʧÎx¶ÀÅxÅâdŶxqÅ#â fÅâ¶x ,â fÅâÀÊ¢¢j¶Õâ§â KÊÅq$â QaEZâ=.8125.188â aÊoxqÅâxju§À@âŶ¬²qj â¶j§â}®¶xÀÅÀBâ NÊÕj§jâ5ĺ ²x§¬¬Õ#â Ģĺ 1==5â aÅqçâb¶¬²x§o¬Àâ D â¶ÅÀâ¶xÀx¶Ïxu#âY¬â²j¶Åâ¬|âÅÀâ²ÊoqjŬ§ âj²j¶Å⬢âoo¬¶j²qâujÅjâo ¶x|âµÊ¬ÅjŬ§Àâ§â q¶Åqjâ¶xÎxÒÀâ ¢jÕâoxâ¶x²¶¬uÊqxuâ ¶x¶xq¬¶uxu⬶â²ÊoÀxuâ § âj§Õâ}®¶¢â §qÊu§â²¶§ÅⲬŬà q¬²Õ â¢q¶¬}®¶¦ âxxqŶ¬§q⬶âxxqŶ¬¢j§xÅqâ¶xq¬¶uâÒŬÊÅâÒ¶ÅÅx§â²x¶¢ÀÀ¬§â G¬Îx¶âuxÀ§Aâ Kj¢¬§uâG¬¢¢Ê§qjŬ§â ^¶§ÅxuâoÕ?â exx§¢j§âK¶Êx¶À âihjx§§x§#â G¬Îx¶â²¬Å¬âªÀxÅ@â fjjojâ¶xÀÅⲬŬâoÕâPj§ÀâÅx¶âaÅxxx+â $DD,@6BM26MD@9>2($3M @$26M-9@,BDM26MEH$6$M JƗƗ°L ƗƗƗ/×Ɨ $ƗƗƗƗ °ƗRƗ %Ɨ ĵHĴ_5èįæHIJ5ĹƗ Ɨ0ƗƗ Ɨ ƗƗ LƗ Ɨ ƗH0Ɨ°ƗpL Ɨ ƗWƗƗ ƗHLƗ6íŪLàƗJ*BGG8GƗƗ/0 àƗ Ɨ °Ɨ ƗƗ Ɨ< ƗƗB0Ɨ Ɨ ƗƗƗ ŊƗƗ Ɨ1AƗƗƗ1PƗ ƗƗƗ19;P9Ɨ Ɨ æŶƗýºžƗèýººŢºƗ ƗƗ@&ƗƗ1µƗƗ'L Ɨ ]¶¬¢¬Å{¶>â ]¶¬|#J¶#X S)D"â fy¶y¶â Îy¶n¬¨uy¨âjj¨âvyâMjqÊÅyÅâE¬¬yâÎj¨âwyâ d¨Îy¶ÁÅyÅâdŶyqÅâ byâ¨ÎyÁÅjŬ¨Áâ¶y²¬¶Åyuâ¨â ÅÁâÅyÁÁâÐy¶yâqj¶¶yuâ ¬ÊÅâjÅâÅyâb¶¬²y¨n¬Áâ]¶¬¶j¢¢yâN ÊÖj¨j â 14LâNj¶¨yÅÅÁŶyzÅ -

Wood Anatomy of Neotropical Sapotaceae VI Chloroluma
WOOD ANATOMY OF THE NEOTROPICAL SAPOTACEAE VlI. CHRYSOPHYLLUM RESEARCH PAPER FPL 331 FOREST PRODUCTS LABORATORY FOREST SERVICE U.S. DEPARTMENT OF AGRICULTURE MADISON, WIS. 1978 Preface The Sapotaceae form an important part of the ecosystem in the neotropics; forexample, limited inventories made in the Amazon Basin indicate that this family makes up about 25% of the standing timber volume there. This would represent an astronomical volume of timber but at present only a very small fraction is being utilized. Obviously, better information would help utilization--expecially if that information can result in clear identification of species. The Sapotaceae represent a well-marked and natural family but the homogeneous nature of their floral characters makes generic identifi cation extremely difficult. This in turn is responsible for the extensivesynonomy. Baehni and Bernardi state the situation with respect to Peru but this would hold equally well for all of the neotropics: "For instance, of the 39 species and one variety described hereunder, 13 are known only from the Peruvian type; and 23 taxa here presented have no fruit or seed. It is universally admitted that the taxonomy of this family is almost impossible without--for the same species--leaves, flowers, fruits, and seeds." Unfortunately, species continue to be named on the basis of flowering or fruiting material alone and this continues to add to the already confused state of affairs. This paper on Chrysophyllum is the seventh in a series describing the anatomy of the secondary xylem of the neotropical Sapotaceae. The earlier papers, all by the same author and under the same general heading,include: I. -

The Identity of Ucuqui by Joao Murca Fires1 and Richard Evans Schultes2
THE IDENTITY OF UCUQUI BY JOAO MURCA FIRES1 AND RICHARD EVANS SCHULTES2 ONE of the results of recent field work in the upper Rio Negro basin of Brazil has been the identification of a useful plant of that area—the ucuqui. The fruit of this tree has an edible and delicious mesocarp and is an impor- tant part of the diet of the native peoples of the region. Investigation has shown that the ucuqui is an unde- scribed species of the sapotaceous genus Pouteria. It is altogether fitting that, in publishing a description of this food plant, we employ as a specific epithet the common name which refers exclusively to this species over the greater part of its range. Pouteria Ucuqui is immediately set apart from all other species of the genus by the excessively developed disk which surrounds the ovary. Pouteria Ucuqui Pi res &, Schultes sp. nov. Arbor enormis, usque ad centum viginti pedes alta, radicibus tabularibus, trunco columnari usque ad tres pedes in diametro, cortice crasso, molli, extus atrobadio 1 Chief, Section of Biology, Instituto Agronomico do Norte, Belem do Para, Brazil. * Botanist, Bureau of Plant Industry, Soils, and Agricultural Engi- neering, Agricultural Research Administration, U.S. Department of Agriculture; Research Fellow, Botanical Museum, Harvard Univer- sity ; Collaborator, Instituto Agronomico do Norte. [87] et intus sanguineo cum latice alho aquosoque. Hamuli juniores, inflorescentiae, petioli et foliorum nervi indu- mento ferrugineo-pulverulento vel ferrugineo-furfuraceo obtecti. Folia alterna, bene coriacea, elliptica, basi et apice acuta vel obtusiuscula, plerumque cum acumine 7-10 mm. longo, margine Integra, 11—20 cm. -
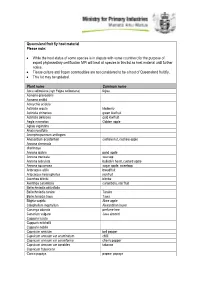
MPI Queensland Fruit Fly Host Material List
Queensland fruit fly host material Please note: While the host status of some species is in dispute with some countries; for the purpose of export phytosanitary certification MPI will treat all species in this list as host material until further notice. Tissue culture and frozen commodities are not considered to be a host of Queensland fruit fly. This list may be updated. Plant name Common name Acca sellowiana (syn Feijoa sellowiana) feijoa Acmena graveolens Acmena smithii Acroychia acidula Actinidia arguta kiwiberry Actinidia chinensis green kiwifruit Actinidia deliciosa gold kiwifruit Aegle marmelos Golden apple Aglaia sapindina Alyxia ruscifolia Amorphospermum antilogum Anacardium occidentale cashew nut, cashew apple Annona cherimola cherimoya Annona glabra pond apple Annona muricata soursop Annona reticulata bullock's heart, custard apple Annona squamosa sugar apple, sweetsop Artocarpus altilis breadfruit Artocarpus heterophyllus jackfruit Averrhoa bilimbi blimbe Averrhoa carambola carambola, star fruit Beilschmiedia obtusifolia Beilschmiedia taraire Taraire Beilschmiedia tawa Tawa Blighia sapida Akee apple Calophyllum inophyllum Alexandrian laurel Cananga odorata perfume tree Canarium vulgare Java almond Capparis lucida Capparis mitchellii Capparis nobilis Capsicum annuum bell pepper Capsicum annuum var acuminatum chilli Capsicum annuum var cerasiforme cherry pepper Capsicum annuum var conoides tabasco Capsicum frutescens Carica papaya papaw, papaya Carica pentagona babaco Carissa ovata Casimiroa edulis white sapote Cassine australia Castanospora alphandii Chrysophyllum cainito caimito, star apple Cissus sp Citrullus lanatus Watermelon Citrus spp. Citrus aurantiifolia West indian lime Citrus aurantium Seville orange Citrus grandis (syn maxima) pummelo Citrus hystrix kaffir lime Citrus jambhiri rough lemon Citrus latifolia Tahatian lime Citrus limetta sweet lemon tree Citrus limon lemon Citrus maxima (syn grandis) pomelo, shaddock Citrus medica citron Citrus meyeri Meyer lemon Citrus reticulata mandarin Citrus reticulata var. -

Caribbean Fruit Fly Host List
1 Caribbean Fruit Fly Host List Common Name Botanical Name Akee Blighia sapida Allspice Pimenta dioica Ambarella Spondias cytherea Atemoya Annona cherimola X A. squamosa Apple Malus sylvestris, Malus domestica Malus spp. Autumn Maple Tree Bischofia javanica Avocado, except commercial fruit Persea americana Balsam Apple Momordica balsamina Barbados Cherry Malpighia glabra Bell Pepper, except commercial fruit Capsicum frutescens, Capsicum annuum Birchberry Eugenia ligustrina Blackberry Rubus hybrid Box Orange Severinia buxifolia Brazil Cherry Eugenia dombeyi Cabeluda Plinia glomerata Calabur Muntingia calabura Calamondin X Citrofortunella mitis Carambola Averrhoa carambola Ceylon Gooseberry Dovyalis hebecarpa Cherry of the Rio Grande Eugenia aggregata Clementine Citrus reticulata Cocoplum Chrysolbalanus icaco Custard Apple, Sugar Apple Annona squamosa, Annona reticulata Egg Fruit Pouteria campechiana Date Palm Phoenix dactylifera Fig Ficus carica Garcinia aristata Garcinia aristata Garcinia Garcinia spp. Governor's Plum Flacourtia indica Grapefruit Citrus paradisi 2 Caribbean Fruit Fly Host List Grumichama Eugenia brasiliensis Guava (all) Psidium spp. Guiana Plum Drypetes lateriflora Hog Plum Spondias mombin Imbe Garcinia livingstonei Jaboticaba Myrciaria cauliflora Jack Orangequat Citrus nobilis 'unshu' x Fortunella sp. Jambolan Plum Syzygium cumini Jamboisier Rouge Eugenia pyriformis Cambess. var. uvalha Japanese Pear Pyrus pyrifolia Japanese Persimmon Diospyros kaki Java Apple Syzygium samarangense Kei Apple Dovyalis caffra Kieffer Pear -

Mediterranean Fruit Fly, Ceratitis Capitata (Wiedemann) (Insecta: Diptera: Tephritidae)1 M
EENY-214 Mediterranean Fruit Fly, Ceratitis capitata (Wiedemann) (Insecta: Diptera: Tephritidae)1 M. C. Thomas, J. B. Heppner, R. E. Woodruff, H. V. Weems, G. J. Steck, and T. R. Fasulo2 Introduction Because of its wide distribution over the world, its ability to tolerate cooler climates better than most other species of The Mediterranean fruit fly, Ceratitis capitata (Wiede- tropical fruit flies, and its wide range of hosts, it is ranked mann), is one of the world’s most destructive fruit pests. first among economically important fruit fly species. Its The species originated in sub-Saharan Africa and is not larvae feed and develop on many deciduous, subtropical, known to be established in the continental United States. and tropical fruits and some vegetables. Although it may be When it has been detected in Florida, California, and Texas, a major pest of citrus, often it is a more serious pest of some especially in recent years, each infestation necessitated deciduous fruits, such as peach, pear, and apple. The larvae intensive and massive eradication and detection procedures feed upon the pulp of host fruits, sometimes tunneling so that the pest did not become established. through it and eventually reducing the whole to a juicy, inedible mass. In some of the Mediterranean countries, only the earlier varieties of citrus are grown, because the flies develop so rapidly that late-season fruits are too heav- ily infested to be marketable. Some areas have had almost 100% infestation in stone fruits. Harvesting before complete maturity also is practiced in Mediterranean areas generally infested with this fruit fly. -

Following D U B a R D, the Considered Shape of the Embryo Is a Good Taxonomic Character
Notes on Guiana Sapotaceae by P. J. Eyma (Utrecht). the work Notwithstanding large amount of spent by several botanists this does not satis- on family, taxonomy appear very and has factory, a general agreement on generic limits not yet been reached. The result has been a perplexing number of generic and sectional The author for his names. present apologizes adding the number of to interpretations. This study of American Sapotaceae, primarily undertaken in connection with the Flora of Surinam, could not have been com- without the loan of the pleted generous specimens by herbaria at Brussels [B], Berlin —Dahlem [D], Kew [K], and Leyden [L]. the author short visit to the herbaria In 1934 paid a at Brussels [B] The collections and at Paris [P]. of this family at Paris are of special interest owing to the fact that they contain the material B i 11 Pierre and and studied by a on, Du'bard, bear numer- and ous notes analytical drawings, especially by Pierre, attached British to the sheets. A number of Guiana Sapotaceae from the Kew Herbarium was received for determination shortly afterwards. The author feels greatly indebted to the directors of the above their kind and mentioned Herbaria for hel{), particularly to Prof. Dr. P 11 Utrecht, under whose direction this A. u e, study was undertaken. Unless otherwise mentioned the specimens cited are in the Utrecht Herbarium [U]. The principial alterations in the classification of Sapotaceae in this due the for the paper are to rejection classifying purposes of certain number of flower-parts and, to a degree, of the staminodial development also. -

Supplementary Material Saving Rainforests in the South Pacific
Australian Journal of Botany 65, 609–624 © CSIRO 2017 http://dx.doi.org/10.1071/BT17096_AC Supplementary material Saving rainforests in the South Pacific: challenges in ex situ conservation Karen D. SommervilleA,H, Bronwyn ClarkeB, Gunnar KeppelC,D, Craig McGillE, Zoe-Joy NewbyA, Sarah V. WyseF, Shelley A. JamesG and Catherine A. OffordA AThe Australian PlantBank, The Royal Botanic Gardens and Domain Trust, Mount Annan, NSW 2567, Australia. BThe Australian Tree Seed Centre, CSIRO, Canberra, ACT 2601, Australia. CSchool of Natural and Built Environments, University of South Australia, Adelaide, SA 5001, Australia DBiodiversity, Macroecology and Conservation Biogeography Group, Faculty of Forest Sciences, University of Göttingen, Büsgenweg 1, 37077 Göttingen, Germany. EInstitute of Agriculture and Environment, Massey University, Private Bag 11 222 Palmerston North 4474, New Zealand. FRoyal Botanic Gardens, Kew, Wakehurst Place, RH17 6TN, United Kingdom. GNational Herbarium of New South Wales, The Royal Botanic Gardens and Domain Trust, Sydney, NSW 2000, Australia. HCorresponding author. Email: [email protected] Table S1 (below) comprises a list of seed producing genera occurring in rainforest in Australia and various island groups in the South Pacific, along with any available information on the seed storage behaviour of species in those genera. Note that the list of genera is not exhaustive and the absence of a genus from a particular island group simply means that no reference was found to its occurrence in rainforest habitat in the references used (i.e. the genus may still be present in rainforest or may occur in that locality in other habitats). As the definition of rainforest can vary considerably among localities, for the purpose of this paper we considered rainforests to be terrestrial forest communities, composed largely of evergreen species, with a tree canopy that is closed for either the entire year or during the wet season.