Ward-Nutrient Composition of American Flamingo Crop Milk.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

6-A John James Audubon, American Flamingo, 1838
JOHN JAMES AUDUBON [1785–1851] 6 a American Flamingo,1838 American Flamingo is one of the 435 hand-colored engravings that River, a major flyway for migratory birds, and eventually wan- make up John James Audubon’s monumental Birds of America, dered farther from home to comb the American frontier for issued in four volumes between 1826 and 1838. The massive unrecorded species. publication includes life-size representations of nearly five hundred Audubon’s procedure was to study and sketch a bird in its natural species of North American birds. Although Audubon was not the habitat before killing it carefully, using fine shot to minimize dam- first to attempt such a comprehensive catalog, his work departed age. His critical innovation was to then thread wire through the from conventional scientific illustration, which showed lifeless spec- specimen, allowing him to fashion a lifelike pose. He worked in imens against a blank background, by presenting the birds as they watercolor, and had completed some four hundred paintings appeared in the wild. When his pictures were first published, when he decided to publish them as a folio of prints. Failing to find some naturalists objected to Audubon’s use of dramatic action and support in Philadelphia, he sailed for England, where he became pictorial design, but these are the qualities that set his work apart lionized as “The American Woodsman.” The engraving firm and make it not only an invaluable record of early American Robert Havell and Son took on the challenge of reproducing wildlife but an unmatched work of American art. Audubon’s paintings on copper plates and tinting the resulting John James Audubon was born in Haiti and educated in France, black-and-white prints by hand. -

A Pliocene Flamingo from Mexico
June, 1944 THE WILSON BULLETIN 77 Vol. 56. No. 2 A PLIOCENE FLAMINGO FROM MEXICO BY LOYE MILLER IELD parties from the California Institute of Technology have been F fortunate in locating a variety of fossil deposits in Mexico that in- cluded bird remains. Some have been very rich in the quantity and variety of material; for example, the San Josecito Cavern of Nuevo Leon (Miller, ,1943), a deposit of Pleistocene age, yielded several thousand bird bones assigned to over forty species. The present paper deals with a collection of ten fragments, all but one of which are in- cluded in a single species. I am indebted to Dr. Chester Stock in charge of the explorations for the opportunity of working with the bird collec- tions. Dr. Alexander Wetmore has loaned comparative material, and Dr. Hildegarde Howard has been a most congenial fellow student during many conferences on the flamingoes, both Recent and Fossil. To these several colleagues my sincere thanks are offered. The ten fragments are from collecting locality No. 289, California Institute of Technology, known. as the Rincon Pliocene, Chihuahua, Mexico. Associated mammal remains include horse, camel, antelope, and carnivore species. The matrix is a fine grained silt of lightest color, without cementing material. A stiff brush serves to remove it from the well petrified bones. Unfortunately the specimens are most frag- mentary. They do, however, prove to be of interest in several respects; most notably they prove (since several speciments are from pre-volant young) that a small speciesof flamingo was present as a breeding bird. This is the earliest record for the family in America. -

Flamingo ABOUT the GROUP
Flamingo ABOUT THE GROUP Bulletin of the IUCN-SSC/Wetlands International The Flamingo Specialist Group (FSG) was established in 1978 at Tour du Valat in France, under the leadership of Dr. Alan Johnson, who coordinated the group until 2004 (see profile at www.wetlands.org/networks/Profiles/January.htm). Currently, the group is FLAMINGO SPECIALIST GROUP coordinated from the Wildfowl & Wetlands Trust at Slimbridge, UK, as part of the IUCN- SSC/Wetlands International Waterbird Network. The FSG is a global network of flamingo specialists (both scientists and non- scientists) concerned with the study, monitoring, management and conservation of the world’s six flamingo species populations. Its role is to actively promote flamingo research and conservation worldwide by encouraging information exchange and cooperation amongst these specialists, and with other relevant organisations, particularly IUCN - SSC, Ramsar, WWF International and BirdLife International. FSG members include experts in both in-situ (wild) and ex-situ (captive) flamingo conservation, as well as in fields ranging from field surveys to breeding biology, diseases, tracking movements and data management. There are currently 165 members around the world, from India to Chile, and from France to South Africa. Further information about the FSG, its membership, the membership list serve, or this bulletin can be obtained from Brooks Childress at the address below. Chair Assistant Chair Dr. Brooks Childress Mr. Nigel Jarrett Wildfowl & Wetlands Trust Wildfowl & Wetlands Trust Slimbridge Slimbridge Glos. GL2 7BT, UK Glos. GL2 7BT, UK Tel: +44 (0)1453 860437 Tel: +44 (0)1453 891177 Fax: +44 (0)1453 860437 Fax: +44 (0)1453 890827 [email protected] [email protected] Eastern Hemisphere Chair Western Hemisphere Chair Dr. -
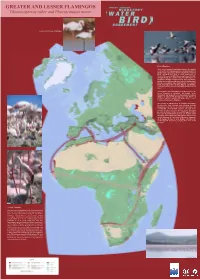
GREATER and LESSER FLAMINGOS Phoenicopterus Ruber and Phoeniconaias Minor
GREATER AND LESSER FLAMINGOS Phoenicopterus ruber and Phoeniconaias minor Greater and Lesser Flamingos © Cliff Buckton © P & H Harris Lesser Flamingo The Lesser Flamingo Phoeniconaias minor is the smallest of the world's five flamingo species. It occurs primarily in the Rift Valley lakes of East Africa with about 4 to 5 million birds estimated, but also in small populations in Namibia/Botswana (40,000), Mauritania/Senegal (15,400), Ethiopia (8,300). The alkaline lakes of the Rift Valley are the primary feeding areas for the East Africa population. During non-breeding periods these lakes often hold almost the entire population. Huge feeding flocks of 1-2 million birds frequently gather on lakes Bogoria and Nakuru, creating one of the most stunning wildlife spectacles in the world. Although it is still the most numerous of the five species, the Lesser Flamingo is classified as globally "near threatened" due primarily to its dependence on a limited number of unprotected breeding sites and threats of proposed soda-ash mining and hydro-electric power schemes on the main breeding lakes. The question of whether there is occasional interchange between the East African and southern African populations has yet to be resolved definitely, but considerable circumstantial evidence has now been assembled to show that East African Lesser Flamingos probably do fly to Botswana to breed during periods when the Lake Makgadikgadi Salt Pans are flooded. Their migration routes, flight range and stopover places (if any) are still unknown. It is now known that Lesser Flamingos do fly during the day, at great heights, well above the normal diurnal movement of eagles, their main aerial predator. -

Flamingo Newsletter 17, 2009
ABOUT THE GROUP The Flamingo Specialist Group (FSG) is a global network of flamingo specialists (both scientists and non-scientists) concerned with the study, monitoring, management and conservation of the world’s six flamingo species populations. Its role is to actively promote flamingo research, conservation and education worldwide by encouraging information exchange and cooperation among these specialists, and with other relevant organisations, particularly the IUCN Species Survival Commission (SSC), the Ramsar Convention on Wetlands, the Convention on Conservation of Migratory Species (CMS), the African-Eurasian Migratory Waterbird Agreement (AEWA), and BirdLife International. The group is coordinated from the Wildfowl & Wetlands Trust, Slimbridge, UK, as part of the IUCN-SSC/Wetlands International Waterbird Network. FSG members include experts in both in-situ (wild) and ex-situ (captive) flamingo conservation, as well as in fields ranging from research surveys to breeding biology, infectious diseases, toxicology, movement tracking and data management. There are currently 286 members representing 206 organisations around the world, from India to Chile, and from France to South Africa. Further information about the FSG, its membership, the membership list serve, or this bulletin can be obtained from Brooks Childress at the address below. Chair Dr. Brooks Childress Wildfowl & Wetlands Trust Slimbridge Glos. GL2 7BT, UK Tel: +44 (0)1453 860437 Fax: +44 (0)1453 860437 [email protected] Eastern Hemisphere Chair Western Hemisphere Chair Dr. Arnaud Béchet Dr. Felicity Arengo Station biologique, Tour du Valat American Museum of Natural History Le Sambuc Central Park West at 79th Street 13200 Arles, France New York, NY 10024 USA Tel : +33 (0) 4 90 97 20 13 Tel: +1 212 313-7076 Fax : +33 (0) 4 90 97 20 19 Fax: +1 212 769-5292 [email protected] [email protected] Citation: Childress, B., Arengo, F. -

1 ID Euring Latin Binomial English Name Phenology Galliformes
BIRDS OF METAURO RIVER: A GREAT ORNITHOLOGICAL DIVERSITY IN A SMALL ITALIAN URBANIZING BIOTOPE, REQUIRING GREATER PROTECTION 1 SUPPORTING INFORMATION / APPENDICE Check list of the birds of Metauro river (mouth and lower course / Fano, PU), up to September 2020. Lista completa delle specie ornitiche del fiume Metauro (foce e basso corso /Fano, PU), aggiornata ad Settembre 2020. (*) In the study area 1 breeding attempt know in 1985, but in particolar conditions (Pandolfi & Giacchini, 1985; Poggiani & Dionisi, 1988a, 1988b, 2019). ID Euring Latin binomial English name Phenology GALLIFORMES Phasianidae 1 03700 Coturnix coturnix Common Quail Mr, B 2 03940 Phasianus colchicus Common Pheasant SB (R) ANSERIFORMES Anatidae 3 01690 Branta ruficollis The Red-breasted Goose A-1 (2012) 4 01610 Anser anser Greylag Goose Mi, Wi 5 01570 Anser fabalis Tundra/Taiga Bean Goose Mi, Wi 6 01590 Anser albifrons Greater White-fronted Goose A – 4 (1986, february and march 2012, 2017) 7 01520 Cygnus olor Mute Swan Mi 8 01540 Cygnus cygnus Whooper Swan A-1 (1984) 9 01730 Tadorna tadorna Common Shelduck Mr, Wi 10 01910 Spatula querquedula Garganey Mr (*) 11 01940 Spatula clypeata Northern Shoveler Mr, Wi 12 01820 Mareca strepera Gadwall Mr, Wi 13 01790 Mareca penelope Eurasian Wigeon Mr, Wi 14 01860 Anas platyrhynchos Mallard SB, Mr, W (R) 15 01890 Anas acuta Northern Pintail Mi, Wi 16 01840 Anas crecca Eurasian Teal Mr, W 17 01960 Netta rufina Red-crested Pochard A-4 (1977, 1994, 1996, 1997) 18 01980 Aythya ferina Common Pochard Mr, W 19 02020 Aythya nyroca Ferruginous -

The Handrearing of a Chilian Flamingo And
£; 'E (j) CJ) :§ o > .0 o (5 .c 0.. PInk Floyd The Handrearing ofa Chilian Flamingo and its Introduction to the Flock Floyd (second from right) seen as a member ofthe flock. He can be distinguished be the light by Chris Smith gray down on the headand neckand by the lighter shade ofpink on the body andwings. Shortly Animal Technician after Floyd was introduced to the flock, several pairs C!f.flamingos successfully hatched and Oklahoma City Zoological Park reared theiryoung. ink Floyd: to many people it is birds care. However, after one of the hatching, chicks stay in or near the the name of a rock band that has first eggs disappeared from the nest, nest for about the first week. After Precorded many cIa sic albums the remaining and subsequent eggs leaving the nest, chicks congregate such as "The Wall" and "The Dark Side were pulled and replaced with dummy with other chicks, forming creches or of the Moon. ' To the personnel at the eggs. The eggs were placed in incuba nursery groups. These creches can Oklahoma City Zoological Park, it is also tor at a temperature of 99.5° F. (37.5° consist of several thousand young the name of its first captive-hatched C). The hygrometer was maintained birds. Chilean Flamingo, Phoenicopterus between 82-84°F (30-31°C). The eggs Adult flamingos take turns watching chilensis. were turned five times daily. A total of over the chicks. While the chicks are in The Oklahoma City Zoo has exhib eight flamingo eggs were pulled from the nursery, the parents continue to ited Chilean Flamingos since 1966, but the nests. -

Modern Birds Classification System Tinamiformes
6.1.2011 Classification system • Subclass: Neornites (modern birds) – Superorder: Paleognathae, Neognathae Modern Birds • Paleognathae – two orders, 49 species • Struthioniformes—ostriches, emus, kiwis, and allies • Tinamiformes—tinamous Ing. Jakub Hlava Department of Zoology and Fisheries CULS Tinamiformes • flightless • Dwarf Tinamou • consists of about 47 species in 9 genera • Dwarf Tinamou ‐ 43 g (1.5 oz) and 20 cm (7.9 in) • Gray Tinamou ‐ 2.3 kg (5.1 lb) 53 cm (21 in) • small fruits and seeds, leaves, larvae, worms, and mollusks • Gray Tinamou 1 6.1.2011 Struthioniformes Struthioniformes • large, flightless birds • Ostrich • most of them now extinct • Cassowary • chicks • Emu • adults more omnivorous or insectivorous • • adults are primarily vegetarian (digestive tracts) Kiwi • Emus have a more omnivorous diet, including insects and other small animals • kiwis eat earthworms, insects, and other similar creatures Neognathae Galloanserae • comprises 27 orders • Anseriformes ‐ waterfowl (150) • 10,000 species • Galliformes ‐ wildfowl/landfowl (250+) • Superorder Galloanserae (fowl) • Superorder Neoaves (higher neognaths) 2 6.1.2011 Anseriformes (screamers) Anatidae (dablling ducks) • includes ducks, geese and swans • South America • cosmopolitan distribution • Small group • domestication • Large, bulky • hunted animals‐ food and recreation • Small head, large feet • biggest genus (40‐50sp.) ‐ Anas Anas shoveler • mallards (wild ducks) • pintails • shlhovelers • wigeons • teals northern pintail wigeon male (Eurasian) 3 6.1.2011 Tadorninae‐ -

Federal Register/Vol. 85, No. 74/Thursday, April 16, 2020/Notices
21262 Federal Register / Vol. 85, No. 74 / Thursday, April 16, 2020 / Notices acquisition were not included in the 5275 Leesburg Pike, Falls Church, VA Comment (1): We received one calculation for TDC, the TDC limit would not 22041–3803; (703) 358–2376. comment from the Western Energy have exceeded amongst other items. SUPPLEMENTARY INFORMATION: Alliance, which requested that we Contact: Robert E. Mulderig, Deputy include European starling (Sturnus Assistant Secretary, Office of Public Housing What is the purpose of this notice? vulgaris) and house sparrow (Passer Investments, Office of Public and Indian Housing, Department of Housing and Urban The purpose of this notice is to domesticus) on the list of bird species Development, 451 Seventh Street SW, Room provide the public an updated list of not protected by the MBTA. 4130, Washington, DC 20410, telephone (202) ‘‘all nonnative, human-introduced bird Response: The draft list of nonnative, 402–4780. species to which the Migratory Bird human-introduced species was [FR Doc. 2020–08052 Filed 4–15–20; 8:45 am]‘ Treaty Act (16 U.S.C. 703 et seq.) does restricted to species belonging to biological families of migratory birds BILLING CODE 4210–67–P not apply,’’ as described in the MBTRA of 2004 (Division E, Title I, Sec. 143 of covered under any of the migratory bird the Consolidated Appropriations Act, treaties with Great Britain (for Canada), Mexico, Russia, or Japan. We excluded DEPARTMENT OF THE INTERIOR 2005; Pub. L. 108–447). The MBTRA states that ‘‘[a]s necessary, the Secretary species not occurring in biological Fish and Wildlife Service may update and publish the list of families included in the treaties from species exempted from protection of the the draft list. -

Population Size and Movements of the Greater Flamingo (Phoenicopterus Roseus) in the Jaffna Peninsula, Sri Lanka: Results from a Long-Term Study
Ceylon Journal of Science 47(4) 2018: 373-378 DOI: http://doi.org/10.4038/cjs.v47i4.7555 RESEARCH ARTICLE Population size and movements of the Greater Flamingo (Phoenicopterus roseus) in the Jaffna peninsula, Sri Lanka: Results from a long-term study Chaminda S. Wijesundara1,*, Saumya Wanniarachchi1, Tharangi Hettiarachchi1, Supun Galappaththi1, Asela Weerawardhana1 and Packiyanathan Rajkumar2,3 1Department of Zoology, University of Peradeniya, Peradeniya, Sri Lanka 2Postgraduate Institute of Science, University of Peradeniya, Peradeniya, Sri Lanka 3Divisional Secretariat, Chundukkuli, Jaffna, Sri Lanka Received:12/05/2018; Accepted:02/08/2018 Abstract: The Greater Flamingo (Phoenicopterus roseus) is an and western Africa, from east Africa to South Africa and uncommon migrant bird species found in Sri Lanka, and is a major Madagascar, and east to Kazakhstan and through Middle attraction among avitourists. Jaffna Peninsula, Mannar Island, and East to India and Sri Lanka (Primack, 2010; del Hoyo et the southeastern coastal areas are the known strongholds of this al., 2017). In Sri Lanka, it is mainly found in the northern species in Sri Lanka. Previous studies on this species in the Jaffna parts of the island (Wijesundara et al., 2017b), where, in Peninsula are limited, most probably due to the inaccessibility some areas such as Jaffna region, it is one of the most of the area during the three-decade long civil war. Hence, the abundant migratory bird species (Wijesundara et al., objectives of the present study were to determine the population 2016). Even though it is generally recognized as a migrant size and movements of the Greater Flamingo in major flocking species, a large number can be seen year-round in the areas in the Jaffna Peninsula. -

Long-Legged Pink Things
nld n hn: Wht r th? Whr d th fr? DAVID S. LEE N.C. State Museum of Natural Sciences P.O. Box 27647, Raleigh, N.C. 27611 Pearson et al. (1942), Sprunt and Chamberlain (1949), and the American Ornithol- ogists' Union Check-list (1957, 1983) consider the records of Greater [American] Flamin- gos 1 in the Carolinas as naturally occurring vagrants. The primary South Carolina records are ones provided by Audubon (1840-1844) and Wayne (1887). The Audubon record is somewhat vague. "A very few of these birds have been known to proceed eastward of the Floridas beyond Charleston in South Carolina, and some have been procured there within eight or ten years back." Wayne's record is of a young, storm-driven male killed on DeBardien Island in September 1876. The specimen was not saved. Sprunt and Chamber- lain (1949) cite an apparent "tongue in cheek" news clipping from the Charleston Courier on 20 July 1818 providing evidence of an even earlier record. It states, "We hope that they [other migrating birds] will meet with better reception than the unfortunate flamingo who recently paid us the honor of a visit from South America, but before he arrived in the metropolis, was slain at John's Island by a man who mistook him for a British soldier." The news article states that the bird was placed in the Charleston Museum, but by 1949 there was no record of its existence. Other records of flamingos available for South Carolina are provided in Table 1. In North Carolina the earliest record was made by the manager of the Pea Island Refuge, Samuel A. -

NEWS and NOTES by Paul Hess
NEWS AND NOTES by Paul Hess First-year Migrants sumably innate southwesterly bearing, and many ended up May Need Experience in Iberia far south of the usual winter range. His report in 1958 suggests that adults improve their inherent naviga - Inheritance or experience? Genetic programming or practi - tional map based on experience, but that first-year birds’ di - cal learning? Various studies show that both inherited and rectional behavior has not progressed beyond an inherited learned information helps birds to migrate to the right rudimentary compass ( Ardea 46:1–37). place in fall and to return to the right place in spring. Im - Fifty years later, Kasper Thorup and six coauthors have mature birds apparently use an innate compass that may added a radio-tracking dimension to similar research using not always allow them to deviate from “hard-wired” direc - White-crowned Sparrows. Results they report in 2007 are a near-perfect analogy to Perdeck’s findings, except that long- distance, non-social, nocturnal migrants are studied rather than short-distance, social, diurnal migrants ( Proceedings of the National Academy of Sciences 104:18115–18119). Fif - teen adult and 15 first-year southbound migrants of the gambelii subspecies were captured at a fall stopover site in Washington state and transported to New Jersey, where each was fitted with a tiny radio transmitter. The birds’ ini - tial movements near the release site were monitored by ground crews, but after flying away from the immediate area (usually at night) they were tracked individually by aircraft for distances as far as 122 kilometers. Adults and immatures rapidly demonstrated a conspicu - ous behavioral difference.