Flamingo Newsletter 17, 2009
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

6-A John James Audubon, American Flamingo, 1838
JOHN JAMES AUDUBON [1785–1851] 6 a American Flamingo,1838 American Flamingo is one of the 435 hand-colored engravings that River, a major flyway for migratory birds, and eventually wan- make up John James Audubon’s monumental Birds of America, dered farther from home to comb the American frontier for issued in four volumes between 1826 and 1838. The massive unrecorded species. publication includes life-size representations of nearly five hundred Audubon’s procedure was to study and sketch a bird in its natural species of North American birds. Although Audubon was not the habitat before killing it carefully, using fine shot to minimize dam- first to attempt such a comprehensive catalog, his work departed age. His critical innovation was to then thread wire through the from conventional scientific illustration, which showed lifeless spec- specimen, allowing him to fashion a lifelike pose. He worked in imens against a blank background, by presenting the birds as they watercolor, and had completed some four hundred paintings appeared in the wild. When his pictures were first published, when he decided to publish them as a folio of prints. Failing to find some naturalists objected to Audubon’s use of dramatic action and support in Philadelphia, he sailed for England, where he became pictorial design, but these are the qualities that set his work apart lionized as “The American Woodsman.” The engraving firm and make it not only an invaluable record of early American Robert Havell and Son took on the challenge of reproducing wildlife but an unmatched work of American art. Audubon’s paintings on copper plates and tinting the resulting John James Audubon was born in Haiti and educated in France, black-and-white prints by hand. -

A Pliocene Flamingo from Mexico
June, 1944 THE WILSON BULLETIN 77 Vol. 56. No. 2 A PLIOCENE FLAMINGO FROM MEXICO BY LOYE MILLER IELD parties from the California Institute of Technology have been F fortunate in locating a variety of fossil deposits in Mexico that in- cluded bird remains. Some have been very rich in the quantity and variety of material; for example, the San Josecito Cavern of Nuevo Leon (Miller, ,1943), a deposit of Pleistocene age, yielded several thousand bird bones assigned to over forty species. The present paper deals with a collection of ten fragments, all but one of which are in- cluded in a single species. I am indebted to Dr. Chester Stock in charge of the explorations for the opportunity of working with the bird collec- tions. Dr. Alexander Wetmore has loaned comparative material, and Dr. Hildegarde Howard has been a most congenial fellow student during many conferences on the flamingoes, both Recent and Fossil. To these several colleagues my sincere thanks are offered. The ten fragments are from collecting locality No. 289, California Institute of Technology, known. as the Rincon Pliocene, Chihuahua, Mexico. Associated mammal remains include horse, camel, antelope, and carnivore species. The matrix is a fine grained silt of lightest color, without cementing material. A stiff brush serves to remove it from the well petrified bones. Unfortunately the specimens are most frag- mentary. They do, however, prove to be of interest in several respects; most notably they prove (since several speciments are from pre-volant young) that a small speciesof flamingo was present as a breeding bird. This is the earliest record for the family in America. -

Climatic Effects on Lake Basins. Part I: Modeling Tropical Lake Levels
15 JUNE 2011 R I C K O E T A L . 2983 Climatic Effects on Lake Basins. Part I: Modeling Tropical Lake Levels MARTINA RICKO AND JAMES A. CARTON Department of Atmospheric and Oceanic Science, University of Maryland, College Park, College Park, Maryland CHARON BIRKETT Earth System Science Interdisciplinary Center, University of Maryland, College Park, College Park, Maryland (Manuscript received 28 December 2009, in final form 9 December 2010) ABSTRACT The availability of satellite estimates of rainfall and lake levels offers exciting new opportunities to estimate the hydrologic properties of lake systems. Combined with simple basin models, connections to climatic variations can then be explored with a focus on a future ability to predict changes in storage volume for water resources or natural hazards concerns. This study examines the capability of a simple basin model to estimate variations in water level for 12 tropical lakes and reservoirs during a 16-yr remotely sensed observation period (1992–2007). The model is constructed with two empirical parameters: effective catchment to lake area ratio and time delay between freshwater flux and lake level response. Rainfall datasets, one reanalysis and two satellite-based observational products, and two radar-altimetry-derived lake level datasets are explored and cross checked. Good agreement is observed between the two lake level datasets with the lowest correlations occurring for the two small lakes Kainji and Tana (0.87 and 0.89). Fitting observations to the simple basin model provides a set of delay times between rainfall and level rise ranging up to 105 days and effective catchment to lake ratios ranging between 2 and 27. -

Flamingo ABOUT the GROUP
Flamingo ABOUT THE GROUP Bulletin of the IUCN-SSC/Wetlands International The Flamingo Specialist Group (FSG) was established in 1978 at Tour du Valat in France, under the leadership of Dr. Alan Johnson, who coordinated the group until 2004 (see profile at www.wetlands.org/networks/Profiles/January.htm). Currently, the group is FLAMINGO SPECIALIST GROUP coordinated from the Wildfowl & Wetlands Trust at Slimbridge, UK, as part of the IUCN- SSC/Wetlands International Waterbird Network. The FSG is a global network of flamingo specialists (both scientists and non- scientists) concerned with the study, monitoring, management and conservation of the world’s six flamingo species populations. Its role is to actively promote flamingo research and conservation worldwide by encouraging information exchange and cooperation amongst these specialists, and with other relevant organisations, particularly IUCN - SSC, Ramsar, WWF International and BirdLife International. FSG members include experts in both in-situ (wild) and ex-situ (captive) flamingo conservation, as well as in fields ranging from field surveys to breeding biology, diseases, tracking movements and data management. There are currently 165 members around the world, from India to Chile, and from France to South Africa. Further information about the FSG, its membership, the membership list serve, or this bulletin can be obtained from Brooks Childress at the address below. Chair Assistant Chair Dr. Brooks Childress Mr. Nigel Jarrett Wildfowl & Wetlands Trust Wildfowl & Wetlands Trust Slimbridge Slimbridge Glos. GL2 7BT, UK Glos. GL2 7BT, UK Tel: +44 (0)1453 860437 Tel: +44 (0)1453 891177 Fax: +44 (0)1453 860437 Fax: +44 (0)1453 890827 [email protected] [email protected] Eastern Hemisphere Chair Western Hemisphere Chair Dr. -

Trinidad & Tobago II 2019 BIRDS
Field Guides Tour Report Trinidad & Tobago II 2019 Dec 27, 2019 to Jan 5, 2020 Tom Johnson For our tour description, itinerary, past triplists, dates, fees, and more, please VISIT OUR TOUR PAGE. Each one of those red dots is a Scarlet Ibis - and look closely, you'll see a strip of pink from the American Flamingo flock at the bottom. This was our evening scene at Caroni Swamp. Photo by group member Delle Daniels. The island nation of Trinidad and Tobago provides an accessible bounty of neotropical wildlife with comfortable accommodation. Our holiday tour this year was no exception – we spent five nights at the famed Asa Wright Nature Centre in Trinidad and two nights on the sandy shores of the Atlantic on Tobago. We drank coffee and tea in the morning as the rising sun brought legions of hummingbirds and honeycreepers to the feeding station below the Asa Wright veranda, studied the mating displays of the odd Bearded Bellbird and transforming White-bearded Manakin, sought the stunning Blue-and- yellow Macaw at Nariva Swamp, enjoyed the waves of brilliant Scarlet Ibis ripple past at Caroni Swamp, and watched with excitement as Magnificent Frigatebirds tail-chased Red-billed Tropicbirds over the blue water below Little Tobago Island. There was something for everyone – for those who were taken by Neotropical birding challenges, there were Chestnut-collared and Lesser Swallow-tailed swifts to pick out of the skies above the Arima Valley and a Gray-throated Leaftosser skulking in the shadows. For those more attracted to spectacles of movement and color, we turned to the diminutive Tufted Coquettes of Asa Wright’s gardens and the jaw-droppingly pink American Flamingos striding below the river of Scarlet Ibis at Caroni Swamp. -

Wetland Birds in the Recent Fossil Record of Britain and Northwest Europe John R
Wetland birds in the recent fossil record of Britain and northwest Europe John R. Stewart 18. Dalmatian Pelican Pelecanus crispus, Deep Bay, Mai Po, Hong Kong, February 1995. Geological evidence suggests that Dalmatian Pelicans bred in Britain, and in other western European countries (including The Netherlands and Denmark), prior to and during the Iron Age. Ray Tipper. ABSTRACT Wetland habitats in Britain and other parts of western Europe have been severely depleted during the latter part of the Holocene owing principally to drainage and land reclamation. Changes in the distribution of a number of wetland bird species can be gauged from archaeological and geological site records of larger birds, whose remains are generally better preserved. Key species are discussed here, including a heron Nycticorax fenensis and a crane Grus primigenia, two extinct species named on possibly uncertain fossil evidence. We can let our minds wander back to the misty realms of fifteen hundred years ago, to a wonderful Britain which was alive with bird song from coast to coast, which sheltered wolves, bears and boars in its dark woodlands, cranes in its marshes, bustards on its heaths and beavers by its streams, and we can visualize the great pink pelican sweeping on its huge pinions over the reedy waterways which then penetrated by secret paths into the very heart of what is now Somerset. (Whitlock, 1953) © British Birds 97 • January 2004 • 33-43 33 Wetland birds in the recent fossil record f all the major habitats in northwest species, including Mute Swan Cygnus olor and Europe, wetlands may have been the Common Crane, may have become physically Omost severely depleted during the smaller owing to habitat impoverishment. -
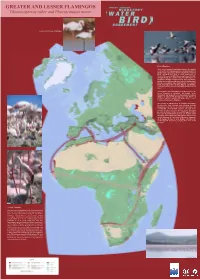
GREATER and LESSER FLAMINGOS Phoenicopterus Ruber and Phoeniconaias Minor
GREATER AND LESSER FLAMINGOS Phoenicopterus ruber and Phoeniconaias minor Greater and Lesser Flamingos © Cliff Buckton © P & H Harris Lesser Flamingo The Lesser Flamingo Phoeniconaias minor is the smallest of the world's five flamingo species. It occurs primarily in the Rift Valley lakes of East Africa with about 4 to 5 million birds estimated, but also in small populations in Namibia/Botswana (40,000), Mauritania/Senegal (15,400), Ethiopia (8,300). The alkaline lakes of the Rift Valley are the primary feeding areas for the East Africa population. During non-breeding periods these lakes often hold almost the entire population. Huge feeding flocks of 1-2 million birds frequently gather on lakes Bogoria and Nakuru, creating one of the most stunning wildlife spectacles in the world. Although it is still the most numerous of the five species, the Lesser Flamingo is classified as globally "near threatened" due primarily to its dependence on a limited number of unprotected breeding sites and threats of proposed soda-ash mining and hydro-electric power schemes on the main breeding lakes. The question of whether there is occasional interchange between the East African and southern African populations has yet to be resolved definitely, but considerable circumstantial evidence has now been assembled to show that East African Lesser Flamingos probably do fly to Botswana to breed during periods when the Lake Makgadikgadi Salt Pans are flooded. Their migration routes, flight range and stopover places (if any) are still unknown. It is now known that Lesser Flamingos do fly during the day, at great heights, well above the normal diurnal movement of eagles, their main aerial predator. -

Comments on the Population Status of Chilean Flamingos at Lagoa Do Peixe National Park, Southern Brazil
Delfino and Aldana-Ardila. Flamingo 2020, pages: 21-26. Comments on the population status of Chilean flamingos at Lagoa do Peixe National Park, Southern Brazil Henrique Cardoso Delfino 1* & Oscar Maurício Aldana-Ardila 1 1 Universidade Federal do Rio Grande do Sul, Instituto de Biociências, Departamento de Zoologia, Programa de Pós-Graduação em Biologia Animal, Laboratório de Ecologia e Sistemática de Aves e Mamíferos Marinhos (LABSMAR). Av. Bento Gonçalves, 9500. CEP: 91509-900, Porto Alegre, RS, Brasil. *Corresponding author: [email protected] Abstract In South America, the Chilean flamingo (Phoenicopterus chilensis) is distributed from south of the Equator to southern Argentina, passing by the Brazilian coast. One of the locations where this species is present in southern Brazil is the Lagoa do Peixe National Park, between the cities of Mostardas and Tavares, in Rio Grande do Sul state. This area is a natural reserve implemented to conserve both coastal biodiversity and the many species of migratory birds that use the area in contranuptial periods. Although the flamingo is well known in the region, there is a lack, in scientific literature, of information about the population of flamingos living inside the park. In this paper, we comment on the current population and conservation status of Chilean flamingos in the Lagoa do Peixe National Park, bringing attention to the necessities to protect the park from political pressures and to increase research activity on these birds in this area. Resumen En América del Sur, el flamenco austral (Phoenicopterus chilensis) se distribuye desde el sur del Ecuador hasta el sur de Argentina, pasando por la costa brasileña. -

Stress-Tolerance and Taxonomy of Culturable Bacterial Communities Isolated from a Central Mojave Desert Soil Sample
geosciences Article Stress-Tolerance and Taxonomy of Culturable Bacterial Communities Isolated from a Central Mojave Desert Soil Sample Andrey A. Belov 1,*, Vladimir S. Cheptsov 1,2 , Elena A. Vorobyova 1,2, Natalia A. Manucharova 1 and Zakhar S. Ezhelev 1 1 Soil Science Faculty, Lomonosov Moscow State University, Moscow 119991, Russia; [email protected] (V.S.C.); [email protected] (E.A.V.); [email protected] (N.A.M.); [email protected] (Z.S.E.) 2 Space Research Institute, Russian Academy of Sciences, Moscow 119991, Russia * Correspondence: [email protected]; Tel.: +7-917-584-44-07 Received: 28 February 2019; Accepted: 8 April 2019; Published: 10 April 2019 Abstract: The arid Mojave Desert is one of the most significant terrestrial analogue objects for astrobiological research due to its genesis, mineralogy, and climate. However, the knowledge of culturable bacterial communities found in this extreme ecotope’s soil is yet insufficient. Therefore, our research has been aimed to fulfil this lack of knowledge and improve the understanding of functioning of edaphic bacterial communities of the Central Mojave Desert soil. We characterized aerobic heterotrophic soil bacterial communities of the central region of the Mojave Desert. A high total number of prokaryotic cells and a high proportion of culturable forms in the soil studied were observed. Prevalence of Actinobacteria, Proteobacteria, and Firmicutes was discovered. The dominance of pigmented strains in culturable communities and high proportion of thermotolerant and pH-tolerant bacteria were detected. Resistance to a number of salts, including the ones found in Martian regolith, as well as antibiotic resistance, were also estimated. -

Lesser Flamingo (Phoeniconaias Minor) Mass-Die Offs
Investigating the dynamics of mosquito vector populations within Lake Ecosystems: The case of East African Rift Valley Lakes Nakuru, Bogoria and Lesser Flamingo (Phoeniconaias minor) mass-die offs. By Juliet Kinyua M.S1, Michael Shiroya Ph.D.1, Anita Kiplagat2, Alice Bett M.S2, Steve Presley Ph.D1. 1Texas Tech University In collaboration with 2The Kenya Wildlife Service Funded by African Bird Club Mosquito vector survey Kenya Abstract Recent changes in the occurrence and spectrum of infectious diseases affecting wildlife have contributed to the growing importance of zoonotic diseases. Emerging infectious diseases in wildlife stem from turbulence in the complex interrelationships between host, pathogen and environment. Much remains unknown about the true prevalence of arboviruses in East Africa and the mosquito vectors responsible for maintenance and virus transmission. Such a deep and thorough representation is needed and will help make better sense of the effect, patterns and prevalence of mosquito vectors and arbovirus networks. This study involved the collection of mosquitoes around the Rift Valley Flamingo lakes Nakuru and Bogoria over a period of one month from December 2012 to January 2013. Using EVS CO2 baited traps we collected a total of 2212 mosquitoes from four genera Culex, Aedes, Mansonia and Anopheles. From this collection 89.9% were from Lake Nakuru and 10% from Lake Bogoria with Culex species being the highest number overall and in Lake Nakuru whereas Aedes species dominated the Lake Bogoria collection. Lake Nakuru had a higher species diversity of the two lakes with a Shannon-Weiner Index value of 1.39 whereas Lake Bogoria had Shannon-Weiner Index value of 0.98. -

Ôø Å Òù× Ö Ôø
ÔØ ÅÒÙ×Ö ÔØ Geology of the Vilama caldera: a new interpretation of a large-scale explosive event in the Central Andean plateau during the Upper Miocene M.M. Soler, P.J Caffe, B.L. Coira, A.T. Onoe, S. Mahlburg Kay PII: S0377-0273(07)00084-4 DOI: doi: 10.1016/j.jvolgeores.2007.04.002 Reference: VOLGEO 3668 To appear in: Journal of Volcanology and Geothermal Research Received date: 27 April 2006 Revised date: 14 November 2006 Accepted date: 11 April 2007 Please cite this article as: Soler, M.M., Caffe, P.J., Coira, B.L., Onoe, A.T., Kay, S. Mahlburg, Geology of the Vilama caldera: a new interpretation of a large-scale explosive event in the Central Andean plateau during the Upper Miocene, Journal of Volcanology and Geothermal Research (2007), doi: 10.1016/j.jvolgeores.2007.04.002 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. ACCEPTED MANUSCRIPT 1 Geology of the Vilama caldera: a new interpretation of a large-scale explosive event in 2 the Central Andean plateau during the Upper Miocene. 3 4 M.M. Solera, P.J Caffea,* , B.L. Coiraa, A.T. Onoeb, S. -

GENOMIC INSIGHTS of an ANDEAN MULTI-RESISTANT SOIL ACTINOBACTERIUM of BIOTECHNOLOGICAL INTEREST Daniel Alonso-Reyes1
bioRxiv preprint doi: https://doi.org/10.1101/2020.12.21.423370; this version posted December 22, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 GENOMIC INSIGHTS OF AN ANDEAN MULTI-RESISTANT SOIL 2 ACTINOBACTERIUM OF BIOTECHNOLOGICAL INTEREST 3 4 Daniel Alonso-Reyes1; Fátima Silvina Galván1, Luciano Raúl Portero1; Natalia Noelia 5 Alvarado1; María Eugenia Farías2; Martín P. Vazquez3; Virginia Helena Albarracín1,4* 6 1Laboratorio de Microbiología Ultraestructural y Molecular, Centro Integral de 7 Microscopía Electrónica (CIME), Facultad de Agronomía y Zootecnia, UNT y 8 CONICET, Tucumán, Argentina 9 2Laboratorio de Investigaciones Microbiológicas de Lagunas Andinas (LIMLA), Planta 10 Piloto de Procesos Industriales y Microbiológicos (PROIMI), CCT, CONICET, 11 Tucumán, Argentina. 12 3HERITAS-CONICET, Ocampo 210 bis, Predio CCT, Rosario, 2000, Santa Fe. 13 4Facultad de Ciencias Naturales e Instituto Miguel Lillo, Universidad Nacional de 14 Tucumán, Tucumán, Argentina. 15 Running headline: ANDEAN MULTI-RESISTANT SOIL ACTINOBACTERIUM 16 Keywords: NESTERENKONIA, SOIL, PUNA, GENOMICS, EXTREMOPHILES, 17 BIOTECHNOLOGY 18 19 *Corresponding author: 20 Virginia Helena Albarracín, Centro Integral de Microscopía Electrónica (CIME, 21 CONICET, UNT) Camino de Sirga s/n. FAZ, Finca El Manantial, Yerba Buena (4107). 22 Tucumán, Argentina. E-mail: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/2020.12.21.423370; this version posted December 22, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity.