Lesser Flamingo (Phoeniconaias Minor) Mass-Die Offs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Flamingo ABOUT the GROUP
Flamingo ABOUT THE GROUP Bulletin of the IUCN-SSC/Wetlands International The Flamingo Specialist Group (FSG) was established in 1978 at Tour du Valat in France, under the leadership of Dr. Alan Johnson, who coordinated the group until 2004 (see profile at www.wetlands.org/networks/Profiles/January.htm). Currently, the group is FLAMINGO SPECIALIST GROUP coordinated from the Wildfowl & Wetlands Trust at Slimbridge, UK, as part of the IUCN- SSC/Wetlands International Waterbird Network. The FSG is a global network of flamingo specialists (both scientists and non- scientists) concerned with the study, monitoring, management and conservation of the world’s six flamingo species populations. Its role is to actively promote flamingo research and conservation worldwide by encouraging information exchange and cooperation amongst these specialists, and with other relevant organisations, particularly IUCN - SSC, Ramsar, WWF International and BirdLife International. FSG members include experts in both in-situ (wild) and ex-situ (captive) flamingo conservation, as well as in fields ranging from field surveys to breeding biology, diseases, tracking movements and data management. There are currently 165 members around the world, from India to Chile, and from France to South Africa. Further information about the FSG, its membership, the membership list serve, or this bulletin can be obtained from Brooks Childress at the address below. Chair Assistant Chair Dr. Brooks Childress Mr. Nigel Jarrett Wildfowl & Wetlands Trust Wildfowl & Wetlands Trust Slimbridge Slimbridge Glos. GL2 7BT, UK Glos. GL2 7BT, UK Tel: +44 (0)1453 860437 Tel: +44 (0)1453 891177 Fax: +44 (0)1453 860437 Fax: +44 (0)1453 890827 [email protected] [email protected] Eastern Hemisphere Chair Western Hemisphere Chair Dr. -
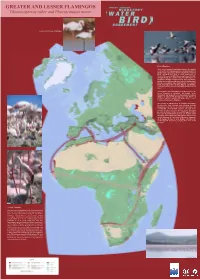
GREATER and LESSER FLAMINGOS Phoenicopterus Ruber and Phoeniconaias Minor
GREATER AND LESSER FLAMINGOS Phoenicopterus ruber and Phoeniconaias minor Greater and Lesser Flamingos © Cliff Buckton © P & H Harris Lesser Flamingo The Lesser Flamingo Phoeniconaias minor is the smallest of the world's five flamingo species. It occurs primarily in the Rift Valley lakes of East Africa with about 4 to 5 million birds estimated, but also in small populations in Namibia/Botswana (40,000), Mauritania/Senegal (15,400), Ethiopia (8,300). The alkaline lakes of the Rift Valley are the primary feeding areas for the East Africa population. During non-breeding periods these lakes often hold almost the entire population. Huge feeding flocks of 1-2 million birds frequently gather on lakes Bogoria and Nakuru, creating one of the most stunning wildlife spectacles in the world. Although it is still the most numerous of the five species, the Lesser Flamingo is classified as globally "near threatened" due primarily to its dependence on a limited number of unprotected breeding sites and threats of proposed soda-ash mining and hydro-electric power schemes on the main breeding lakes. The question of whether there is occasional interchange between the East African and southern African populations has yet to be resolved definitely, but considerable circumstantial evidence has now been assembled to show that East African Lesser Flamingos probably do fly to Botswana to breed during periods when the Lake Makgadikgadi Salt Pans are flooded. Their migration routes, flight range and stopover places (if any) are still unknown. It is now known that Lesser Flamingos do fly during the day, at great heights, well above the normal diurnal movement of eagles, their main aerial predator. -

Flamingo Newsletter 17, 2009
ABOUT THE GROUP The Flamingo Specialist Group (FSG) is a global network of flamingo specialists (both scientists and non-scientists) concerned with the study, monitoring, management and conservation of the world’s six flamingo species populations. Its role is to actively promote flamingo research, conservation and education worldwide by encouraging information exchange and cooperation among these specialists, and with other relevant organisations, particularly the IUCN Species Survival Commission (SSC), the Ramsar Convention on Wetlands, the Convention on Conservation of Migratory Species (CMS), the African-Eurasian Migratory Waterbird Agreement (AEWA), and BirdLife International. The group is coordinated from the Wildfowl & Wetlands Trust, Slimbridge, UK, as part of the IUCN-SSC/Wetlands International Waterbird Network. FSG members include experts in both in-situ (wild) and ex-situ (captive) flamingo conservation, as well as in fields ranging from research surveys to breeding biology, infectious diseases, toxicology, movement tracking and data management. There are currently 286 members representing 206 organisations around the world, from India to Chile, and from France to South Africa. Further information about the FSG, its membership, the membership list serve, or this bulletin can be obtained from Brooks Childress at the address below. Chair Dr. Brooks Childress Wildfowl & Wetlands Trust Slimbridge Glos. GL2 7BT, UK Tel: +44 (0)1453 860437 Fax: +44 (0)1453 860437 [email protected] Eastern Hemisphere Chair Western Hemisphere Chair Dr. Arnaud Béchet Dr. Felicity Arengo Station biologique, Tour du Valat American Museum of Natural History Le Sambuc Central Park West at 79th Street 13200 Arles, France New York, NY 10024 USA Tel : +33 (0) 4 90 97 20 13 Tel: +1 212 313-7076 Fax : +33 (0) 4 90 97 20 19 Fax: +1 212 769-5292 [email protected] [email protected] Citation: Childress, B., Arengo, F. -

Common Birds of Namibia and Botswana 1 Josh Engel
Common Birds of Namibia and Botswana 1 Josh Engel Photos: Josh Engel, [[email protected]] Integrative Research Center, Field Museum of Natural History and Tropical Birding Tours [www.tropicalbirding.com] Produced by: Tyana Wachter, R. Foster and J. Philipp, with the support of Connie Keller and the Mellon Foundation. © Science and Education, The Field Museum, Chicago, IL 60605 USA. [[email protected]] [fieldguides.fieldmuseum.org/guides] Rapid Color Guide #584 version 1 01/2015 1 Struthio camelus 2 Pelecanus onocrotalus 3 Phalacocorax capensis 4 Microcarbo coronatus STRUTHIONIDAE PELECANIDAE PHALACROCORACIDAE PHALACROCORACIDAE Ostrich Great white pelican Cape cormorant Crowned cormorant 5 Anhinga rufa 6 Ardea cinerea 7 Ardea goliath 8 Ardea pupurea ANIHINGIDAE ARDEIDAE ARDEIDAE ARDEIDAE African darter Grey heron Goliath heron Purple heron 9 Butorides striata 10 Scopus umbretta 11 Mycteria ibis 12 Leptoptilos crumentiferus ARDEIDAE SCOPIDAE CICONIIDAE CICONIIDAE Striated heron Hamerkop (nest) Yellow-billed stork Marabou stork 13 Bostrychia hagedash 14 Phoenicopterus roseus & P. minor 15 Phoenicopterus minor 16 Aviceda cuculoides THRESKIORNITHIDAE PHOENICOPTERIDAE PHOENICOPTERIDAE ACCIPITRIDAE Hadada ibis Greater and Lesser Flamingos Lesser Flamingo African cuckoo hawk Common Birds of Namibia and Botswana 2 Josh Engel Photos: Josh Engel, [[email protected]] Integrative Research Center, Field Museum of Natural History and Tropical Birding Tours [www.tropicalbirding.com] Produced by: Tyana Wachter, R. Foster and J. Philipp, -

Flamingo Atlas
Flamingo Bulletin of the IUCN-SSC/Wetlands International FLAMINGO SPECIALIST GROUP Number 15, December 2007 ISSN 1680-1857 ABOUT THE GROUP The Flamingo Specialist Group (FSG) was established in 1978 at Tour du Valat in France, under the leadership of Dr. Alan Johnson, who coordinated the group until 2004. Currently, the group is coordinated from the Wildfowl & Wetlands Trust at Slimbridge, UK, as part of the IUCN-SSC/Wetlands International Waterbird Network. The FSG is a global network of flamingo specialists (both scientists and non- scientists) involved in the study, monitoring, management and conservation of the world’s six flamingo species populations. Its role is to actively promote flamingo research and conservation worldwide by encouraging information exchange and cooperation among these specialists, and with other relevant organisations, particularly IUCN - SSC, Wetlands International, Ramsar, Convention on the Conservation of Migratory Species, African Eurasian Migratory Waterbird Agreement, and BirdLife International. FSG members include experts in both in-situ (wild) and ex-situ (captive) flamingo conservation, as well as in fields ranging from field surveys to breeding biology, infectious diseases, toxicology, movement tracking and data management. There are currently 208 members around the world, from India to Chile, and from Finland to South Africa. Further information about the FSG, its membership, the membership list serve, or this bulletin can be obtained from Brooks Childress at the address below. Chair Assistant Chair Dr. Brooks Childress Mr. Nigel Jarrett Wildfowl & Wetlands Trust Wildfowl & Wetlands Trust Slimbridge Slimbridge Glos. GL2 7BT, UK Glos. GL2 7BT, UK Tel: +44 (0)1453 860437 Tel: +44 (0)1453 891177 Fax: +44 (0)1453 860437 Fax: +44 (0)1453 890827 [email protected] [email protected] Eastern Hemisphere Chair Western Hemisphere Chair Dr. -

The Relevance of Captive Flamingos to Meeting the Four Aims of the Modern Zoo Paul E
Rose. Flamingo 2018, pages: 23-32 The relevance of captive flamingos to meeting the four aims of the modern zoo Paul E. Rose Centre for Research in Animal Behaviour, Washington Singer Labs, University of Exeter, Perry Road, Exeter, Devon, EX4 4QG, UK / WWT, Slimbridge Wetland Centre, Slimbridge, Gloucestershire, GL2 7BT, UK. For correspondence: [email protected] Abstract Flamingos are popular inhabitants of zoological collections around the world and are one of the most commonly-seen zoo species. The modern zoo has four aims: Conservation, education, research and recreation. Meeting each of these aims adds value to the animal collection and allows zoos to explain their wider work to their visitors and guests. As many zoos are reliant on gate entry and return visitation as their main source of income, the animal collection must maintain visitor interest and be engaging. The income from visitors is used by zoos to uphold their education, conservation and research programmes, and the way in which animals are displayed to the visitor helps to define educational strategies and impart relevant information on a species, its ecosystem, ecology and conservation value. As zoos move towards encouraging behaviour change in their visitors, stories on climate change and human impacts can be emphasised by using particular species within the animal collection to tell such stories. Iconic or eye-catching species can have a particular role in encouraging visitors to remember stories on human impacts and their effects on the planet. This paper outlines the ways in which zoo-housed flamingos can be utilised to emphasis the main roles of the modern zoo and provides a discussion of the relevance of zoo-housed birds to meeting the wider aims of the zoo (both in terms of its work with other zoos and that with the wild world). -

Lesser Flamingo Phoeniconaias Minor
NEAR THREATENED | FLAMINGO 305 Tanzania, Etosha Pan, Namibia and Sua Pan, Botswana (Berry 1972, Hancock and Liversedge 1990, Simmons 1996, Lesser Flamingo Simmons 1997d, McCulloch and Irvine 2004). During four Phoeniconaias minor consecutive years, from 2007 to 2011, Lesser Flamingos bred on a purpose-built, S-shaped, 25x250 m island constructed at Kamfers Dam, just north of Kimberley (Northern Cape, 2015 Regional Status Near Threatened [A2c+3c+4c] South Africa) (Anderson 2008, Anderson and Anderson 2010). An estimated 24 000 chicks were produced during 2000 Regional Status Near Threatened [A1c; A2c] these breeding events, representing the only documented 2015 Global Status Near Threatened [A2c+3c+4c] incident of successful breeding in South Africa. The island Status change reason Not applicable flooded in the summer of 2011/2012 and no breeding has Family name Phoenicopteridae subsequently taken place (Anderson and Anderson 2012). At Species name author (Geoffroy Saint-Hilaire É, 1798) least 12 breeding attempts at sites in South Africa have been unsuccessful (Uys and Macleod 1967, Brooke 1984), mainly Population size c. 120 000 individuals because the sites are small, with low numbers of flamingos, (southern Africa) and therefore unsuitable for mass breeding (Simmons 1996, Distribution size (AoO) 1 204 km2 Simmons 1997d). Regional endemic No The Lesser Flamingo’s non-breeding distribution in South Africa is centred on the central Highveld, but it also occurs along the West and South coasts (Borello et al. 1998). Large numbers have been recorded at Lake St Lucia, KwaZulu-Na- JUSTIFICATION tal (IBA SA058), Berg River (IBA SA104), Wadrif Saltpan The Lesser Flamingo Phoeniconaias minor is classified as and Langebaan Lagoon (IBA SA105) in Western Cape, and globally Near Threatened due to a perceived decline in popu- Kamfers Dam, Northern Cape (IBA SA032) (Taylor 1999). -

Tanzania National Single Species Action Plan 2010-2020 for the Conservation of the Lesser Flamingo (Phoeniconaias Minor)
THE UNITED REPUBLIC OF TANZANIA MINISTRY OF NATURAL RESOURCES AND TOURISM WILDLIFE DIVISION Sustainable Wetlands Management Program Tanzania National Single Species Action Plan 2010-2020 for the Conservation of the Lesser Flamingo (Phoeniconaias minor) (Photo courtesy Ma Aeberhard) February 2010 For the establishment of Community Based Natural Resource Management of Wetlands Tanzania Na onal Single Species Ac on Plan 2010-2020 for the Conserva on of the Lesser Flamingo (Phoeniconaias minor) 1 THE UNITED REPUBLIC OF TANZANIA MINISTRY OF NATURAL RESOURCES AND TOURISM WILDLIFE DIVISION Sustainable Wetlands Management Program Tanzania National Single Species Action Plan 2010-2020 for the Conservation of the Lesser Flamingo (Phoeniconaias minor) February 2010 For the establishment of Community Based Natural Resource Management of Wetlands Tanzania Na onal Single Species Ac on Plan 2010-2020 for the Conserva on of the Lesser Flamingo (Phoeniconaias minor) © Wildlife Division, 2011 For further informa on, please contact: Director Wildlife, Wildlife Division, PO Box 1994, Dar es Salaam, Tanzania. Phone: 255 22 2866408, 2866376, 2866418. Fax: 255 22 2865836, 2863496.Foreword E-mail: [email protected]., ii Tanzania Na onal Single Species Ac on Plan 2010-2020 for the Conserva on of the Lesser Flamingo (Phoeniconaias minor) Acknowledgements The Director of Wildlife wishes to acknowledge the following: Prepared with Financial and Technical Assistance from: Danida support to Sustainable Wetlands Management Program (SWMP), helped fund the mee ngs, workshops and prin ng of the fi rst dra and publica on. Flamingo Land, supported the publica on of the fi nal document. The support from BirdLife Interna onal for the par cipa on of regional specialists to a end mee ngs, is highly appreciated. -

Lake Nakuru Flamingoes
www.saltworkconsultants.com Salty MattersJohn Warren - Friday, May 23, 2016 Lake Nakuru flamingoes– Life’s response to feast and famine in schizohaline lacus- trine hydrologies The Flamingo Connection Nakuru, leaving enough pink carcases to spur an interna- tional newspaper to describe the lake as a “flamingo death An aviator once described Lake Nakuru as “a crucible of camp.” Two years prior, 43,800 of the birds had perished pink and crimson fire,” with a million flamingos painting at Tanzania’s Lake Manyara, the first major die-off docu- an astonishing band of colour that burst into pieces as the mented at that alkaline, soda-rich lake. Previous mass die- birds took flight (Figure 1). offs occurred at Lake Nakuru and two other Kenyan lakes Flamingo population levels in Lake Nakuru and any mass in 1993, 1995, and 1997, as well as at two lakes in Tanzania “die-offs” are popularly considered as indicators of the en- in 2002. At the same time, birds were gathering in places vironmental health of Nakuru and other lakes in the Af- they have never been documented before. Since 2006 there rican rift valley with significant flamingo populations. In have been additional population crashes at Nakuru and El- 2006, more than 30,000 of the birds were found dead at mentia (Table 1). Figure 1. Flamingo ock feeding on the edge of Lake Nakuru (full-size image can be downloaded from http://www.yannarthusber- trand2.org) Page 1 www.saltworkconsultants.com Date of crash Lake Reference where these birds dominate the macrofauna in 1971 Elmenteita Melack and Kilham 1974 some modern saline lakes, are described by Scott 1973 Nakuru Vareschi et al. -

ECOLOGY of LESSER FLAMINGOS Phoeniconaias Minor (Geoffroy, 1798) in the MOMELLA LAKES, ARUSHA NATIONAL PARK - TANZANIA
ECOLOGY OF LESSER FLAMINGOS Phoeniconaias minor (Geoffroy, 1798) IN THE MOMELLA LAKES, ARUSHA NATIONAL PARK - TANZANIA By Deogratias Ladislaus Lihepanyama BSc. Hons (University of Dar es Salaam, Tanzania) A THESIS SUBMITTED TO THE SCHOOL OF BIOLOGICAL SCIENCES, UNIVERSITY OF NAIROBI, IN PARTIAL FULFILMENT OF THE DEGREE OF MASTER OF SCIENCE IN BIOLOGY OF CONSERVATION 2016 DECLARATION This work is my original research work and has not been presented for a degree or any other award in any other University. Deogratias Ladislaus Lihepanyama I56/79610/2012 Signature…………………………………… Date………………………………… Declaration by supervisors This thesis has been submitted with our approval as University Supervisors. Dr. John Maina Githaiga Senior Lecturer School of Biological Sciences University of Nairobi P. O. Box 30197 – 00100 NAIROBI Signature…………………………………………. Date……………………………………… Dr. Francis Mwaura Senior Lecturer Department of Geography & Environmental Studies University of Nairobi P. O. Box 30197 – 00100 NAIROBI Signature……………………………………… Date…………………………………….... ii DEDICATION This work is dedicated to my parents Mr. and Mrs. Ladislaus Lihepanyama for their prayers, support and encouragement during the entire period of my studies. I highly appreciate their concern. iii TABLE OF CONTENTS Content Page DECLARATION ............................................................................................................................... ii DEDICATION ................................................................................................................................. -

Journal of Threatened Taxa
The Journal of Threatened Taxa (JoTT) is dedicated to building evidence for conservaton globally by publishing peer-reviewed artcles OPEN ACCESS online every month at a reasonably rapid rate at www.threatenedtaxa.org. All artcles published in JoTT are registered under Creatve Commons Atributon 4.0 Internatonal License unless otherwise mentoned. JoTT allows unrestricted use, reproducton, and distributon of artcles in any medium by providing adequate credit to the author(s) and the source of publicaton. Journal of Threatened Taxa Building evidence for conservaton globally www.threatenedtaxa.org ISSN 0974-7907 (Online) | ISSN 0974-7893 (Print) Note Photographic record of Lesser Flamingo Phoeniconaias minor (Aves: Phoenicopteridae) in Ramganga river, Bareilly, India Pichaimuthu Gangaiamaran, Afab A. Usmani, G.V. Gopi, S.A. Hussain & Khursid A. Khan 26 July 2021 | Vol. 13 | No. 8 | Pages: 19159–19161 DOI: 10.11609/jot.7375.13.8.19159-19161 For Focus, Scope, Aims, and Policies, visit htps://threatenedtaxa.org/index.php/JoTT/aims_scope For Artcle Submission Guidelines, visit htps://threatenedtaxa.org/index.php/JoTT/about/submissions For Policies against Scientfc Misconduct, visit htps://threatenedtaxa.org/index.php/JoTT/policies_various For reprints, contact <[email protected]> The opinions expressed by the authors do not refect the views of the Journal of Threatened Taxa, Wildlife Informaton Liaison Development Society, Zoo Outreach Organizaton, or any of the partners. The journal, the publisher, the host, and the part- Publisher & Host -

Enclosure Alterations for Improved Lesser Flamingo Health and Welfare at the Oregon
Suhn. Flamingo 2020, pages: 31-35. Enclosure alterations for improved lesser flamingo health and welfare at the Oregon Zoo Barbara Suhn 1 * 1 Bird Department, Oregon Zoo, 4001 SW Canyon Road, Portland, Oregon, 97221, USA. *Corresponding author: [email protected] Abstract Flamingos in captivity are prone to pathological changes to foot health and condition, commonly identified as pododermatitis (“bumblefoot”). Lesser flamingos at the Oregon Zoo experienced fluctuations in foot health between winter and summer, with severe changes over winter that showed limited improvement in the summer. The zoo embarked on an enclosure alteration project, changing substrates and heating the water of the flamingos’ pool. These changes have markedly improved the health and condition of the birds’ feet, as evidenced by regular monitoring and documentation of foot scores. This article outlines the changes that were undertaken to improve flamingo welfare and explains how the enclosure and the birds are managed to keep a check on their foot condition. Resumen Los flamencos en cautiverio son propensos a condiciones patológicas en la salud y el estado de las patas, comúnmente identificadas como pododermatitis (dermatitis plantar). Los flamencos enanos (Phoeniconaias minor) en el Zoológico de Oregon, EE.UU. experimentaron fluctuaciones en la salud de las patas entre el invierno y el verano, con cambios severos durante el invierno que mostraron una mejora limitada en el verano. El zoológico se embarcó en un proyecto de modificación del recinto, cambiando sustratos y calentando el agua de la piscina de los flamencos. Estos cambios han mejorado notablemente la salud y el estado de las patas de las aves, demostrado en el seguimiento y la documentación regular de las puntuaciones de la planta del pie.