Lake Nakuru Flamingoes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Historical Volcanism and the State of Stress in the East African Rift System
Historical volcanism and the state of stress in the East African Rift System Article Accepted Version Open Access Wadge, G., Biggs, J., Lloyd, R. and Kendall, J.-M. (2016) Historical volcanism and the state of stress in the East African Rift System. Frontiers in Earth Science, 4. 86. ISSN 2296- 6463 doi: https://doi.org/10.3389/feart.2016.00086 Available at http://centaur.reading.ac.uk/66786/ It is advisable to refer to the publisher’s version if you intend to cite from the work. See Guidance on citing . To link to this article DOI: http://dx.doi.org/10.3389/feart.2016.00086 Publisher: Frontiers media All outputs in CentAUR are protected by Intellectual Property Rights law, including copyright law. Copyright and IPR is retained by the creators or other copyright holders. Terms and conditions for use of this material are defined in the End User Agreement . www.reading.ac.uk/centaur CentAUR Central Archive at the University of Reading Reading’s research outputs online 1 Historical volcanism and the state of stress in the East African 2 Rift System 3 4 5 G. Wadge1*, J. Biggs2, R. Lloyd2, J-M. Kendall2 6 7 8 1.COMET, Department of Meteorology, University of Reading, Reading, UK 9 2.COMET, School of Earth Sciences, University of Bristol, Bristol, UK 10 11 * [email protected] 12 13 14 Keywords: crustal stress, historical eruptions, East African Rift, oblique motion, 15 eruption dynamics 16 17 18 19 20 21 Abstract 22 23 Crustal extension at the East African Rift System (EARS) should, as a tectonic ideal, 24 involve a stress field in which the direction of minimum horizontal stress is 25 perpendicular to the rift. -

Flamingo ABOUT the GROUP
Flamingo ABOUT THE GROUP Bulletin of the IUCN-SSC/Wetlands International The Flamingo Specialist Group (FSG) was established in 1978 at Tour du Valat in France, under the leadership of Dr. Alan Johnson, who coordinated the group until 2004 (see profile at www.wetlands.org/networks/Profiles/January.htm). Currently, the group is FLAMINGO SPECIALIST GROUP coordinated from the Wildfowl & Wetlands Trust at Slimbridge, UK, as part of the IUCN- SSC/Wetlands International Waterbird Network. The FSG is a global network of flamingo specialists (both scientists and non- scientists) concerned with the study, monitoring, management and conservation of the world’s six flamingo species populations. Its role is to actively promote flamingo research and conservation worldwide by encouraging information exchange and cooperation amongst these specialists, and with other relevant organisations, particularly IUCN - SSC, Ramsar, WWF International and BirdLife International. FSG members include experts in both in-situ (wild) and ex-situ (captive) flamingo conservation, as well as in fields ranging from field surveys to breeding biology, diseases, tracking movements and data management. There are currently 165 members around the world, from India to Chile, and from France to South Africa. Further information about the FSG, its membership, the membership list serve, or this bulletin can be obtained from Brooks Childress at the address below. Chair Assistant Chair Dr. Brooks Childress Mr. Nigel Jarrett Wildfowl & Wetlands Trust Wildfowl & Wetlands Trust Slimbridge Slimbridge Glos. GL2 7BT, UK Glos. GL2 7BT, UK Tel: +44 (0)1453 860437 Tel: +44 (0)1453 891177 Fax: +44 (0)1453 860437 Fax: +44 (0)1453 890827 [email protected] [email protected] Eastern Hemisphere Chair Western Hemisphere Chair Dr. -
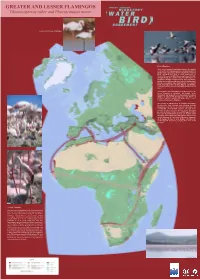
GREATER and LESSER FLAMINGOS Phoenicopterus Ruber and Phoeniconaias Minor
GREATER AND LESSER FLAMINGOS Phoenicopterus ruber and Phoeniconaias minor Greater and Lesser Flamingos © Cliff Buckton © P & H Harris Lesser Flamingo The Lesser Flamingo Phoeniconaias minor is the smallest of the world's five flamingo species. It occurs primarily in the Rift Valley lakes of East Africa with about 4 to 5 million birds estimated, but also in small populations in Namibia/Botswana (40,000), Mauritania/Senegal (15,400), Ethiopia (8,300). The alkaline lakes of the Rift Valley are the primary feeding areas for the East Africa population. During non-breeding periods these lakes often hold almost the entire population. Huge feeding flocks of 1-2 million birds frequently gather on lakes Bogoria and Nakuru, creating one of the most stunning wildlife spectacles in the world. Although it is still the most numerous of the five species, the Lesser Flamingo is classified as globally "near threatened" due primarily to its dependence on a limited number of unprotected breeding sites and threats of proposed soda-ash mining and hydro-electric power schemes on the main breeding lakes. The question of whether there is occasional interchange between the East African and southern African populations has yet to be resolved definitely, but considerable circumstantial evidence has now been assembled to show that East African Lesser Flamingos probably do fly to Botswana to breed during periods when the Lake Makgadikgadi Salt Pans are flooded. Their migration routes, flight range and stopover places (if any) are still unknown. It is now known that Lesser Flamingos do fly during the day, at great heights, well above the normal diurnal movement of eagles, their main aerial predator. -

Lake Turkana and the Lower Omo the Arid and Semi-Arid Lands Account for 50% of Kenya’S Livestock Production (Snyder, 2006)
Lake Turkana & the Lower Omo: Hydrological Impacts of Major Dam & Irrigation Development REPORT African Studies Centre Sean Avery (BSc., PhD., C.Eng., C. Env.) © Antonella865 | Dreamstime © Antonella865 Consultant’s email: [email protected] Web: www.watres.com LAKE TURKANA & THE LOWER OMO: HYDROLOGICAL IMPACTS OF MAJOR DAM & IRRIGATION DEVELOPMENTS CONTENTS – VOLUME I REPORT Chapter Description Page EXECUTIVE(SUMMARY ..................................................................................................................................1! 1! INTRODUCTION .................................................................................................................................... 12! 1.1! THE(CONTEXT ........................................................................................................................................ 12! 1.2! THE(ASSIGNMENT .................................................................................................................................. 14! 1.3! METHODOLOGY...................................................................................................................................... 15! 2! DEVELOPMENT(PLANNING(IN(THE(OMO(BASIN ......................................................................... 18! 2.1! INTRODUCTION(AND(SUMMARY(OVERVIEW(OF(FINDINGS................................................................... 18! 2.2! OMO?GIBE(BASIN(MASTER(PLAN(STUDY,(DECEMBER(1996..............................................................19! 2.2.1! OMO'GIBE!BASIN!MASTER!PLAN!'!TERMS!OF!REFERENCE...........................................................................19! -

Flamingo Newsletter 17, 2009
ABOUT THE GROUP The Flamingo Specialist Group (FSG) is a global network of flamingo specialists (both scientists and non-scientists) concerned with the study, monitoring, management and conservation of the world’s six flamingo species populations. Its role is to actively promote flamingo research, conservation and education worldwide by encouraging information exchange and cooperation among these specialists, and with other relevant organisations, particularly the IUCN Species Survival Commission (SSC), the Ramsar Convention on Wetlands, the Convention on Conservation of Migratory Species (CMS), the African-Eurasian Migratory Waterbird Agreement (AEWA), and BirdLife International. The group is coordinated from the Wildfowl & Wetlands Trust, Slimbridge, UK, as part of the IUCN-SSC/Wetlands International Waterbird Network. FSG members include experts in both in-situ (wild) and ex-situ (captive) flamingo conservation, as well as in fields ranging from research surveys to breeding biology, infectious diseases, toxicology, movement tracking and data management. There are currently 286 members representing 206 organisations around the world, from India to Chile, and from France to South Africa. Further information about the FSG, its membership, the membership list serve, or this bulletin can be obtained from Brooks Childress at the address below. Chair Dr. Brooks Childress Wildfowl & Wetlands Trust Slimbridge Glos. GL2 7BT, UK Tel: +44 (0)1453 860437 Fax: +44 (0)1453 860437 [email protected] Eastern Hemisphere Chair Western Hemisphere Chair Dr. Arnaud Béchet Dr. Felicity Arengo Station biologique, Tour du Valat American Museum of Natural History Le Sambuc Central Park West at 79th Street 13200 Arles, France New York, NY 10024 USA Tel : +33 (0) 4 90 97 20 13 Tel: +1 212 313-7076 Fax : +33 (0) 4 90 97 20 19 Fax: +1 212 769-5292 [email protected] [email protected] Citation: Childress, B., Arengo, F. -

Alcolapia Grahami ERSS
Lake Magadi Tilapia (Alcolapia grahami) Ecological Risk Screening Summary U.S. Fish & Wildlife Service, March 2015 Revised, August 2017, October 2017 Web Version, 8/21/2018 1 Native Range and Status in the United States Native Range From Bayona and Akinyi (2006): “The natural range of this species is restricted to a single location: Lake Magadi [Kenya].” Status in the United States No records of Alcolapia grahami in the wild or in trade in the United States were found. The Florida Fish and Wildlife Conservation Commission has listed the tilapia Alcolapia grahami as a prohibited species. Prohibited nonnative species (FFWCC 2018), “are considered to be dangerous to the ecology and/or the health and welfare of the people of Florida. These species are not allowed to be personally possessed or used for commercial activities.” Means of Introductions in the United States No records of Alcolapia grahami in the United States were found. 1 Remarks From Bayona and Akinyi (2006): “Vulnerable D2 ver 3.1” Various sources use Alcolapia grahami (Eschmeyer et al. 2017) or Oreochromis grahami (ITIS 2017) as the accepted name for this species. Information searches were conducted under both names to ensure completeness of the data gathered. 2 Biology and Ecology Taxonomic Hierarchy and Taxonomic Standing According to Eschmeyer et al. (2017), Alcolapia grahami (Boulenger 1912) is the current valid name for this species. It was originally described as Tilapia grahami; it has also been known as Oreoghromis grahami, and as a synonym, but valid subspecies, of -

Case Study Report For
Case Study One Indigenous Peoples’ Rights in the Kenya Lake System in the Great Rift Valley 1 By DR. KANYINKE SENA Indigenous Peoples’ Rights in the Kenya Lake System in the Great Rift Valley 1 CASE STUDY ONE Indigenous Peoples’ Rights in the Kenya World Heritage (IIPFWH), as a standing global Lake System in the Great Rift Valley body aimed at representing indigenous peo- ples voices in the World Heritage Committee processes.5 The Committee referred to the establishment of the IIPFWH, “As an impor- tant reflection platform on the involvement of Indigenous Peoples in the identification, conservation and management of World Heritage properties, with a particular focus on the nomination process.” 6 Pursuant to the mandate of the Forum, this report aims at analyzing Indigenous Peoples’ involvement in the Kenya Lakes System in the Great Rift Valley World Heritage Site. The report is as result of extensive literature re- view and interviews with communities in and around the lakes that comprise the Kenya K. Sena: Lake Bogoria Lakes System. The Kenya Lake System in the Great Rift Val- ley is a World Heritage site in Kenya which comprises three inter-linked, relatively shal- low, alkaline lakes and their surrounding territories. The lakes system includes Lakes Elementeita, Nakuru and Bogoria in the Rift Valley. The lakes cover a total area of 32,034 and was inscribed as a world heritage site in 2011. The inscription was based on the lakes system outstanding universal values and criterion (vii), (ix) and (x) as provided for, under paragraph 77 of the Operational Guidelines for the Implementation of the World Heritage Convention. -

Lesser Flamingo (Phoeniconaias Minor) Mass-Die Offs
Investigating the dynamics of mosquito vector populations within Lake Ecosystems: The case of East African Rift Valley Lakes Nakuru, Bogoria and Lesser Flamingo (Phoeniconaias minor) mass-die offs. By Juliet Kinyua M.S1, Michael Shiroya Ph.D.1, Anita Kiplagat2, Alice Bett M.S2, Steve Presley Ph.D1. 1Texas Tech University In collaboration with 2The Kenya Wildlife Service Funded by African Bird Club Mosquito vector survey Kenya Abstract Recent changes in the occurrence and spectrum of infectious diseases affecting wildlife have contributed to the growing importance of zoonotic diseases. Emerging infectious diseases in wildlife stem from turbulence in the complex interrelationships between host, pathogen and environment. Much remains unknown about the true prevalence of arboviruses in East Africa and the mosquito vectors responsible for maintenance and virus transmission. Such a deep and thorough representation is needed and will help make better sense of the effect, patterns and prevalence of mosquito vectors and arbovirus networks. This study involved the collection of mosquitoes around the Rift Valley Flamingo lakes Nakuru and Bogoria over a period of one month from December 2012 to January 2013. Using EVS CO2 baited traps we collected a total of 2212 mosquitoes from four genera Culex, Aedes, Mansonia and Anopheles. From this collection 89.9% were from Lake Nakuru and 10% from Lake Bogoria with Culex species being the highest number overall and in Lake Nakuru whereas Aedes species dominated the Lake Bogoria collection. Lake Nakuru had a higher species diversity of the two lakes with a Shannon-Weiner Index value of 1.39 whereas Lake Bogoria had Shannon-Weiner Index value of 0.98. -

Omenda Geothermal Heat Sources in East Africa Rift System 2016
Heat Sources for Geothermal Systems in East African Rift System Peter Omenda Classification by Temperature Volcano hosted Geothermal Systems • Typical model for volcano hosted geothermal system Volcano hosted Geothermal Systems-Menengai • Associated with main rift volcanoes Menengai Geothermal field • Temperatures of > 400oC measured • Shallow magma bodies ~2.3km Geophysical Model across Menengai • Seismics show high velocity under volcano • Common feature of all volcanoes in EARS rift axis MEQ Studies T o B MN15 a Marigat MN13 r in g Coffee o Farm MN12 Bahati ru Majani Mingi ru hu ya 9985000 N To MN14 • Studies revealed that 9980000 Kabarak s s s MN09 Bahati g g g brittle ductile transition MN10 n n n i i i CALDERA Settlement h h h t t t r r r o o o MN11 N N N MN07 To d d d i i i E r r r l MN08 do G G G re zone under Menengai is 9975000 t MN06 Residential Area MN04 at 6km depth T o Nairobi 9970000 NAKURU MN05 MN03URBAN AREA Lake Rongai Nakuru Lake Nakuru Farms National Park MN02 MN01 165000 170000 175000 180000 Grid Eastings (m) NorthWest Ol Rongai Men. Caldera SouthEast 0 -2 ) ) m m K K ( ( h h t t -4 p p e e D D -6 -8 165000 167500 170000 172500 175000 177500 180000 Distance (M) Menengai Stratigraphy • Stratigraphy dominated by trachytes and pyroclastics • Magma body at ~2.3km • Syenitic cap present • Tmax 400oC Menengai stratigraphic Model Tuff layers Trachyte Syenite Magma Menengai Model 200 isotherm 250 isotherm 350 isotherm Magma Olkaria Volcanic Complex • The heat source at Olkaria is associated with – discrete magma chambers that underlie the volcanic centres Main Ethiopian Rift Recent volcano tectonic activity is mainly within the axis. -

Analysis and Mapping of Water-Related Conflicts in the Catchment of Lake Naivasha (Kenya)
Competition over water resources: analysis and mapping of water-related conflicts in the catchment of Lake Naivasha (Kenya) Carolina Boix Fayos February 2002 Competition over water resources: analysis and mapping of water-related conflicts in the catchment of Lake Naivasha (Kenya) By Carolina Boix Fayos Supervisors: Dr. M.McCall (Social Sciences) Drs. J. Verplanke (Social Sciences) Drs. R. Becht (Water Resources) Thesis submitted to the International Institute for Geoinformation Science and Earth Observation in partial fulfilment of the requirements for the degree of Master of Science in Water Resources and Environmental Management Degree Assessment Board Chairman: Prof. Dr. A.M.J. Meijerink (Water Resources) External examiner: Prof. A. van der Veen (University of Twente) Members: Dr. M.K. McCall (Social Sciences) Drs. J.J. Verplanke (Social Sciences) Drs. R. Becht (Water Resources) INTERNATIONAL INSTITUTE FOR GEOINFORMATION SCIENCE AND EARTH OBSERVATION ENSCHEDE, THE NETHERLANDS Disclaimer This document describes work undertaken as part of a programme of study at the International Institute for Geoinformation Science and Earth Observation. All views and opinions expressed therein remain the sole responsibility of the author, and do not necessarily represent those of the institute. A mi abuelo Paco (Francisco Fayos Artés) que me enseñó a apreciar la tierra y sus gentes y a disfrutar con la Geografía y la Historia ACKNOWLEDGEMENT The experience of ITC has been very special. I am very grateful to the Fundación Alfonso Martín Escudero (Madrid, Spain) who paid the ITC fees and supported me economically during the whole period. I am also very grateful to my supervisors Dr. Mike McCall, Drs. -

Direct Utilization of Geothermal Resources in Kenya
Proceedings World Geothermal Congress 2010 Bali, Indonesia, 25-29 April 2010 Direct Utilization of Geothermal Resources in Kenya John Lagat Kenya Electricity Generating Company Ltd, P. O. Box 785 Naivasha 20117 E-mail: [email protected] Keywords: Geothermal, direct use, balneology, space 38oE heating, greenhouses, agriculture, aquaculture, industrial North Island processes 4oN L. Turkana ETHIOPIA ABSTRACT Central Island Geothermal resources in Kenya are mainly located along the Kenyan Rift, which is part of the eastern arm of the South Island African Rift. The primary forms of utilization of Cenozoic Barrier Volcano geothermal energy in Kenya currently are mainly electric volcanics power generation (168 MWe) and direct uses (~18 MWt) to UGANDA Namarunu a small extent. Though about 99% of geothermal utilization in the country is generation of electricity, direct uses are Emuruangogolak gaining momentum in various parts of the country. Farmers Mt. Elgon Silali have for decades, used geothermal heat to dry pyrethrum Paka KENYA Korosi flowers and condense steam for drinking at Eburru while L. Baringo Nyambeni the Oserian Development Company in Naivasha is using Arus-Bogoria geothermal energy to heat 50 hectares of greenhouses of cut Kisumu Oo roses for export. A tourist hotel at Lake Bogoria is utilizing Menengai Mt. Kenya spring water at 38°C to heat a spa pool. Despite the Eburru availability and enormous potential in direct use L. Naivasha applications, little use has been made of low enthalpy fluids Olkaria Longonot in Kenya. This paper therefore discusses direct use Suswa applications and available opportunities in swimming, Ol'Esakut Nairobi bathing, balneology, agricultural, aquiculture and in Olorgesaille o residential and industrial sectors L. -
In Water Composition Due to Abstraction of Soda Ash. Impact
Chapter 6 - Lake Natron Soda Ash ESIA 6 - 8 in water composition due to abstraction of soda ash. Impact: Introduction of animal pests and pathogens to the Lake system Impact No. B/E 3 Changes in disease vector populations Ranking: Negative slight Characteristics: Domestic waste attracting pests. Abandoned borrow pits providing mosquito breeding sites. Increase in introduction of vectors through increased human and vehicle movement. Impact: Loss of fresh water habitats in the Lake due to dry season abstraction Impact No. B/E 4 Changes in aquatic biota Ranking: Negative slight Characteristics: Abstraction of surface water from the Wosi Wosi River Impact: The Cyperus laevigatus sedgelands surrounding the semi sodic springs in the southern and eastern sides of the Lake form critical late dry season grazing for domestic stock and wildlife. Increased pressure or disturbance could deplete the remaining wildlife populations Impact No. B/E 5 Changes in terrestrial plant populations Ranking: Negative moderate Characteristics: Access road along east side of the Lake would threaten the use of 400 ha of dry season grazing. This area has a stocking rate of at least 2.5 LSU/ha during he dry season Impact: Introduction of alien invasive plant and animal species Impact No. B/E 5 Changes in terrestrial plant (and animal) populations Ranking: Negative slight Characteristics: Concerns relating to construction activities, the development of domestic gardens, the introduction of brine shrimp into the process Impact: Illegal hunting activities will increase with human immigration into the area Impact No. B/E 6 Changes in terrestrial wildlife populations Ranking: Negative slight Characteristics: There is a present decline in rare wildlife species such as gerenuk and Coir bustard and local extinction of rhino and Oryx due to increased pressure on grazing resources and increased poaching.