Presidential Green Chemistry Challenge Awards Program: Summary of 2016 Award Entries and Recipients
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Project Leader Lissa Mccracken Michael Simpson, PE Acting Director Sr
Project Leader Lissa McCracken Michael Simpson, PE Acting Director Sr. Environmental Engineer Kentucky Pollution Prevention Center City of Los Angeles University of Louisville Department of Public Works Louisville, KY Bureau of Sanitation Industrial Waste Management Division Cam Metcalf (former Director of Kentucky Los Angeles, CA Pollution Prevention Center) National Pollution Prevention Round 2001 Lancashire Ave #208 Table Board Member Louisville, KY Authors * Editors Michelle Butler, PhD* Michelle Butler, PhD* Sr. Pollution Prevention Engineer Sr. Pollution Prevention Engineer NY State Pollution Prevention Institute NY State Pollution Prevention Institute Rochester Institute of Technology Rochester Institute of Technology Rochester, New York Rochester, New York Al Innes Jonathan M. Rivin, PhD* Minnesota Pollution Control Agency Waste Management Specialist Green Chemistry Coordinator UW Extension-Solid & Hazardous Waste Pollution Prevention Program Education Center St. Paul, MN University of Wisconsin-Stevens Point Stevens Point, WI Lin Kaatz Chary, PhD, MPH Executive Director Cathy Bouge Great Lakes Green Chemistry Network Washington State Gary, IN Department of Ecology Olympia, WA Olga Krel, MS Plancheck Engineer City of Los Angeles Department of Public Works Bureau of Sanitation Industrial Waste Management Division Los Angeles, CA Ally LaTourelle, Esq. Managing Partner BioEconomy Partners Philadelphia, PA *Editors Green Chemistry Guide This manual provides state agencies and technical assistance providers with tools and resources -

Worker and Environmentalist Green Chemistry Awareness Training Curriculum
Worker and Environmentalist Green Chemistry Awareness Training Curriculum The New England Consortium University of Massachusetts Lowell Grant funded by The National Institute of Environmental Health Sciences Grant No. 3U45ES006172-18S2, titled: Administrative Supplements to Promote Partnerships For Environmental Public Health.” This Page Intentionally Left Blank ACKNOWLEDGMENTS Principal Investigator: Craig Slatin, Sc.D., MPH Professor Department of Community Health and Sustainability Director, Center for Health Promotion and Research School of Health and Environment University of Massachusetts Lowell Project Director: Paul Morse, MA, BS Project Manager of The New England Consortium Center for Health Promotion and Research School of Health and Environment University of Massachusetts Lowell This curriculum was a collaboration by the following individuals and institutions: Curriculum Coordinator: Tolle Graham, Labor and Environment Coordinator, Massachusetts Coalition for Occupational Safety & Health (MassCOSH) Curriculum Development Team: Melissa Coffin, Research Associate, Lowell Center for Sustainable Production Amy Cannon, Executive Director, Beyond Benign Foundation Claudie Grout, ENVISION Exceptional Instruction Tom Estabrook, Project Manager - Special Projects, The New England Consortium, UMass Lowell Craig Slatin, Professor, Principal Investigator, The New England Consortium, UMass Lowell Joel Tickner, Associate Professor, Project Director – Lowell Center for Sustainable Production, UMass Lowell The curriculum development team wants -

Cancer Drug Pharmacology Table
CANCER DRUG PHARMACOLOGY TABLE Cytotoxic Chemotherapy Drugs are classified according to the BC Cancer Drug Manual Monographs, unless otherwise specified (see asterisks). Subclassifications are in brackets where applicable. Alkylating Agents have reactive groups (usually alkyl) that attach to Antimetabolites are structural analogues of naturally occurring molecules DNA or RNA, leading to interruption in synthesis of DNA, RNA, or required for DNA and RNA synthesis. When substituted for the natural body proteins. substances, they disrupt DNA and RNA synthesis. bendamustine (nitrogen mustard) azacitidine (pyrimidine analogue) busulfan (alkyl sulfonate) capecitabine (pyrimidine analogue) carboplatin (platinum) cladribine (adenosine analogue) carmustine (nitrosurea) cytarabine (pyrimidine analogue) chlorambucil (nitrogen mustard) fludarabine (purine analogue) cisplatin (platinum) fluorouracil (pyrimidine analogue) cyclophosphamide (nitrogen mustard) gemcitabine (pyrimidine analogue) dacarbazine (triazine) mercaptopurine (purine analogue) estramustine (nitrogen mustard with 17-beta-estradiol) methotrexate (folate analogue) hydroxyurea pralatrexate (folate analogue) ifosfamide (nitrogen mustard) pemetrexed (folate analogue) lomustine (nitrosurea) pentostatin (purine analogue) mechlorethamine (nitrogen mustard) raltitrexed (folate analogue) melphalan (nitrogen mustard) thioguanine (purine analogue) oxaliplatin (platinum) trifluridine-tipiracil (pyrimidine analogue/thymidine phosphorylase procarbazine (triazine) inhibitor) -

To Undergraduate Studies in Chemistry, Chemical Engineering, and Chemical Biology College of Chemistry, University of California, Berkeley, 2011-12
Guide to Undergraduate Studies in Chemistry, Chemical Engineering, and -2012 Chemical Biology College of Chemistry 2011 University of California, Berkeley Academic Calendar 2011-12 Fall Semester 2011 Tele-BEARS Begins April 11 Monday Fee Payment Due August 15 Monday Fall Semester Begins August 18 Thursday Welcome Events August 22-26 Monday-Friday Instruction Begins August 25 Thursday Labor Day Holiday September 5 Monday Veterans Day Holiday November 11 Friday Thanksgiving Holiday November 24-25 Thursday-Friday Formal Classes End December 2 Friday Reading/Review/Recitation Week December 5-9 Monday-Friday Final Examinations December 12-16 Monday-Friday Fall Semester Ends December 16 Friday Winter Holiday December 26-27 Monday-Tuesday New Year’s Holiday December 29-30 Thursday-Friday Spring Semester 2012 Tele-BEARS Begins October 17, 2011 Monday Spring Semester Begins January 10 Tuesday Fee Payment Due January 15 Sunday Martin Luther King Jr. Holiday January 16 Monday Instruction Begins January 17 Tuesday Presidents’ Day Holiday February 20 Monday Spring Recess March 26-30 Monday-Friday César Chávez Holiday March 30 Friday Cal Day To Be Determined Formal Classes End April 27 Friday Reading/Review/Recitation Week April 30-May 4 Monday-Friday Final Examinations May 7-11 Monday-Friday Spring Semester Ends May 11 Friday Summer Sessions 2012 Tele-BEARS Begins February 6 Monday First Six-Week Session May 21-June 29 Monday-Friday Memorial Day Holiday May 28 Monday Ten-Week Session June 4-August 10 Monday-Friday Eight-Week Session June 18-August -

The Role of Green Chemistry in Sustainability
American Chemical Society The Role of Green Chemistry in Sustainability Mary M. Kirchhoff EPA Region 6 QA Conference 19 October 2015 Green Chemistry Green chemistry is the design of chemical products and processes that reduce or eliminate the use and generation of hazardous substances. American Chemical Society 2 Sustainable Development Development that meets the needs of the present without compromising the ability of future generations to meet their own needs. Brundtland Commission American Chemical Society 3 Presidential Green Chemistry Challenge Awards • The Presidential Green Chemistry Challenge was established to recognize and promote fundamental and innovative chemical technologies that accomplish pollution prevention through source reduction and that are useful to industry. American Chemical Society 4 Award Categories • Greener synthetic pathways • Greener reaction conditions • Design of greener chemicals • Small business • Academic • Specific environmental benefit: Climate change American Chemical Society 5 2015 Award Winner • Ethanol and green crude from algae – Bio-engineered cyanobacteria – High conversion (80%) to ethanol – Ethanol purified via Vapor Compression Steam Stripping – Residual biomass converted to green crude – 70% reduction in CO2 emissions relative to gasoline Algenol American Chemical Society 6 12 Principles 1. Prevention 2. Atom economy 3. Less hazardous chemical syntheses 4. Designing safer chemicals 5. Safer solvents and auxiliaries 6. Energy efficiency 7. Renewable feedstocks 8. Reduce derivatives 9. Catalysis 10.Design for degradation 11.Real-time analysis for pollution prevention 12.Inherently safer chemistry American Chemical Society 7 Principle 1 It is better to prevent waste than to treat or clean up waste after it is formed. American Chemical Society 8 E-factor • Weight of byproducts/weight of desired product – Oil refining 0.1 – Bulk chemicals <15 – Fine chemicals 5-50 – Pharmaceuticals 25-100+ Sheldon, ChemTech, 1994, 24, 38. -

Importance of Green Chemistry in Oxidation and Reduction
International Journal of Engineering and Technical Research (IJETR) ISSN: 2321-0869 (O) 2454-4698 (P) Volume-7, Issue-7, July 2017 Importance of Green chemistry in oxidation and reduction Richa Khare, Avidha Kulshrestha, Jaya Pandey, Nidhi Singh Abstract– ”Green chemistry‘ the self-explanatory term is a to equip the students with the required set of tools, their branch of chemistry that involves the application of chemical knowledge and the experience to work with it. product and processes in such a way that reduces the generation This helps us in maintaining and enhancing the high quality of of hazardous chemical waste and its release into the provisions of the green and sustainable chemistry to enable environment. It is an intelligent way of doing chemistry in change to low carbon and bio based economy which is which the waste production is minimized, energy is saved and the depletion of natural resources is cut down to some extent. developed on the high quality of pure and transitional Significant efforts have been made towards the development research, its education and the training. Its networking and of new Green Technologies. It‘s Eenefits should reach to the partnerships which is in the framework of the development common man. We can synthesize many organic molecules with (5). the help of green chemistry methods. In this article we are trying to study about and utilization of green chemistry in different Green Chemistry: A Contribution for Sustainable processes mainly oxidation and reduction processes. Development of Society Green chemistry has contributed a lot in the cleansing the Index Terms– Green Synthesis, Green Chemistry, environment and making our planet a beautiful place to live in Oxidation, Reduction .green chemistry has optimized global mass in order to minimize waste. -

The Effect of High-Dose Thiotepa, Alone Or in Combination with Other
Bone Marrow Transplantation (2007) 40, 891–896 & 2007 Nature Publishing Group All rights reserved 0268-3369/07 $30.00 www.nature.com/bmt ORIGINAL ARTICLE The effect of high-dose thiotepa, alone or in combination with other chemotherapeutic agents, on a murine B-cell leukemia model simulating autologous stem cell transplantation A Abdul-Hai, L Weiss, D Ergas, IB Resnick, S Slavin and MY Shapira Department of Bone Marrow Transplantation and Cancer Immunotherapy, Hadassah–Hebrew University Medical Center, Jerusalem, Israel The use of thiotepa (TH) is increasing, especially in stem Introduction cell transplantation, mainly due to its safety and blood– brain barrier penetration. We evaluated the use of TH in a Thiotepa (TH, triethylene thiophosphoramide), an ethylene murine model simulating autologous stem cell transplan- amide, developed by Lederle Laboratories in 1952, tation, with or without additional agents. Between 1 and possesses mechlorethamine-like alkylating activity and 11 days following inoculation of BALB/c mice with 105– has been used clinically for over 35 years.1 It is of particular 108 B-cell leukemia (BCL1) cells (simulating pre-trans- use in breast cancer, mainly as second-line treatment.2 plant leukemia loads), each group received an ‘induction- TH has been given intrathecally for carcinomatous like’ irradiation and/or cytotoxic regimen. Animals were meningitis, and intravesically for bladder carcinoma.3,4 either followed without treatment, or an adoptive transfer Mild-to-moderate activity was observed in several solid (AT) was performed to untreated BALB/c mice. Admi- tumors and hematological malignancies.1,5 There has nistered alone without AT, high-dose TH did not change been, however, a sustained interest in defining clinical roles the time to appearance of leukemia. -

SUMMARY of PARTICULARLY HAZARDOUS SUBSTANCES (By
SUMMARY OF PARTICULARLY HAZARDOUS SUBSTANCES (by alpha) Key: SC -- Select Carcinogens RT -- Reproductive Toxins AT -- Acute Toxins SA -- Readily Absorbed Through the Skin DHS -- Chemicals of Interest Revised: 11/2012 ________________________________________________________ ___________ _ _ _ _ _ _ _ _ _ _ _ ||| | | | CHEMICAL NAME CAS # |SC|RT| AT | SA |DHS| ________________________________________________________ ___________ | _ | _ | _ | _ | __ | | | | | | | 2,4,5-T 000093-76-5 | | x | | x | | ABRIN 001393-62-0 | | | x | | | ACETALDEHYDE 000075-07-0 | x | | | | | ACETAMIDE 000060-35-5 | x | | | | | ACETOHYDROXAMIC ACID 000546-88-3 ||x| | x | | ACETONE CYANOHYDRIN, STABILIZED 000075-86-5 | | | x | | x | ACETYLAMINOFLUORENE,2- 000053-96-3 | x | | | | | ACID MIST, STRONG INORGANIC 000000-00-0 | x | | | | | ACROLEIN 000107-02-8 | | x | x | x | | ACRYLAMIDE 000079-06-1 | x | x | | x | | ACRYLONITRILE 000107-13-1 | x | x | x | x | | ACTINOMYCIN D 000050-76-0 ||x| | x | | ADIPONITRILE 000111-69-3 | | | x | | | ADRIAMYCIN 023214-92-8 | x | | | | | AFLATOXIN B1 001162-65-8 | x | | | | | AFLATOXIN M1 006795-23-9 | x | | | | | AFLATOXINS 001402-68-2 | x | | x | | | ALL-TRANS RETINOIC ACID 000302-79-4 | | x | | x | | ALPRAZOMAN 028981-97-7 | | x | | x | | ALUMINUM PHOSPHIDE 020859-73-8 | | | x | | x | AMANTADINE HYDROCHLORIDE 000665-66-7 | | x | | x | | AMINO-2,4-DIBROMOANTHRAQUINONE 000081-49-2 | x | | | | | AMINO-2-METHYLANTHRAQUINONE, 1- 000082-28-0 | x | | | | | AMINO-3,4-DIMETHYL-3h-IMIDAZO(4,5f)QUINOLINE,2- 077094-11-2 | x | | | | | AMINO-3,8-DIMETHYL-3H-IMIDAZO(4,5-f)QUINOXALINE, -
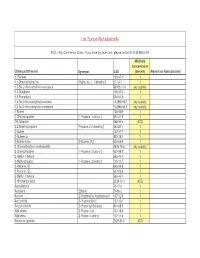
Homeland Security List
List Sorted Alphabetically ACG = Any Commercial Grade. If you have any questions, please contact EHS @ 898-5126. Minimum Concentration Chemical Of Interest Synonym CAS (percent) Amount on Hand (pounds) 1- Pentene 109-67-1 1 1,1-Dimethylhydrazine [Hydrazine, 1, 1-dimethyl-] 57-14-7 1 1,3-Bis(2-chloroethylthio)-n-propane 63905-10-2 any quantity 1,3-Butadiene 106-99-0 1 1,3-Pentadiene 504-60-9 1 1,4-Bis(2-chloroethylthio)-n-butane 142868-93-7 any quantity 1,5-Bis(2-chloroethylthio)-n-pentane 142868-94-8 any quantity 1-Butene 106-98-9 1 1-Chloropropylene [1-Propene, 1-chloro-] 590-21-6 1 1H-Tetrazole 288-94-8 ACG 2,2-Dimethylpropane [Propane, 2,2-dimethyl-] 463-82-1 1 2-Butene 107-01-7 1 2-Butene-cis 590-18-1 1 2-Butene-trans [2-Butene, (E)] 624-64-6 1 2-Chloroethylchloro-methylsulfide 2625-76-5 any quantity 2-Chloropropylene [1-Propene, 2-chloro-] 557-98-2 1 2-Methyl-1-butene 563-46-2 1 2-Methylpropene [1-Propene, 2-methyl-] 115-11-7 1 2-Pentene, (E)- 646-04-8 1 2-Pentene, (Z)- 627-20-3 1 3-Methyl-1-butene 563-45-1 1 5-Nitrobenzotriazol 2338-12-7 ACG Acetaldehyde 75-07-0 1 Acetylene [Ethyne] 74-86-2 1 Acrolein [2-Propenal] or Acrylaldehyde 107-02-8 1 Acrylonitrile [2-Propenenitrile] 107-13-1 1 Acrylyl chloride [2-Propenoyl chloride] 814-68-6 1 Allyl alcohol [2-Propen-1-ol] 107-18-6 1 Allylamine [2-Propen-1-amine] 107-11-9 1 Aluminum (powder) 7429-90-5 ACG Ammonia (anhydrous) 7664-41-7 1 Ammonia (conc. -

Early Industrial Roots of Green Chemistry - II
Firenze University Press www.fupress.com/substantia Feature Article Early Industrial Roots of Green Chemistry - II. International “Pollution Prevention” Efforts Citation: M.A. Murphy (2020) Early Industrial Roots of Green Chemistry - During the 1970’s and 1980’s II. International “Pollution Prevention” Efforts During the 1970’s and 1980’s. Substantia 4(2): 15-57. doi: 10.13128/ Substantia-894 Mark A. Murphy Received: Apr 03, 2020 UVLAW Patents LLC, 171 China Creek Rd., Blowing Rock North Carolina 28605, USA E-mail: [email protected] Revised: May 15, 2020 Just Accepted Online: May 30, 2020 Abstract. Many literature articles and/or conventional histories of “Green Chemis- try” describe its start as being a result of actions at the US Environmental Protection Published: Sep 12, 2020 Agency (“EPA”) and/or in Academia during the 1990’s. But many examples of environ- Copyright: © 2020 M.A. Murphy. This mentally friendly Real-World chemical processes were invented, developed and com- is an open access, peer-reviewed arti- mercialized in the oil refining, commodity chemical, and consumer product industries cle published by Firenze University starting about the time of World War II. Those efforts dramatically accelerated and Press (http://www.fupress.com/substan- evolved into explicitly environmentally oriented “Pollution Prevention” efforts during tia) and distributed under the terms the 1970’s and 1980’s. A UN conference in November 1976 brought together over 150 of the Creative Commons Attribution attendees from industry, academia, and governmental and non-governmental organiza- License, which permits unrestricted tions from 30 countries to address environmental issues related to preventing pollution use, distribution, and reproduction caused by the chemically-related industries. -

Green and Sustainable Chemistry Education: Nurturing a New Generation of Chemists
Green and sustainable chemistry education: Nurturing a new generation of chemists Foundation Paper for GCO II Part IV 23 January 2019 Vania Zuin, Departamento de Quimica, Universidade Federal de Sao Carlos, Brazil Ingo Eilks, Universität Bremen, Institute for Science Education Disclaimer The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the United Nations Environment Programme concerning the legal status of any country, territory, city or area or of its authorities, or concerning delimitation of its frontiers or boundaries. Moreover, the views expressed do not necessarily represent the decision or the stated policy of the United Nations Environment Programme, nor does citing of trade names or commercial processes constitute endorsement. 1 Contents 1. A new way of teaching chemistry ......................................................................................................... 1 2. Education reform gaining momentum in many countries, but some regions lagging behind ............. 5 3. Overcoming barriers: key determinants for effective educational reform ........................................ 12 4. Options for action ............................................................................................................................... 15 References .................................................................................................................................................. 15 2 1. A new way of teaching -

A Green Alternative for Obtaining Potentially Active Compounds †
Proceedings Eco-Friendly Catalytic Aminoselenation of Alkenes: A Green Alternative for Obtaining Potentially Active Compounds † Luana S. Gomes 1,*, Rafaella G. Angelino 1, José S. S. Neto 2, Iris di Leo 3, Claudio Santi 3 and Vanessa Nascimento 1 1 Supraselen Laboratory, Department of Organic Chemistry, Federal Fluminense University (UFF), Rio de Janeiro 24220-008, Brazil; [email protected] (R.G.A.); [email protected] (V.N.) 2 Department of Organic Chemistry, Federal University of Santa Catarina (UFSC), Florianópolis, Santa Catarina 88040-900, Brazil; [email protected] 3 Department of Pharmaceutical Sciences, University of Perugia, 06125 Perugia, Italy; [email protected] (I.d.L.); [email protected] (C.S.) * Correspondence: [email protected] † Presented at the 1st International Electronic Conference on Catalysis Sciences, 10–30 November 2020; Available online: https://eccs2020.sciforum.net. Published: 9 November 2020 Abstract: In this work, a new ecological approach to the selenofunctionalization of alkenes has been described using I2 as catalyst, DMSO as oxidant, under microwave irradiation (MW) in a solvent- and metal-free method. The general idea is to combine organoselenium compounds and triazole nuclei to obtain molecules capable of becoming a powerful class due to their potential pharmacological activity. However, most methods that involve the functionalization of alkenes are generally mediated by the use of transition metals or reagents in large stoichiometric quantities. Thus, the development of direct, clean and environmentally appropriate procedures, which are in accordance with the principles of green chemistry, for the synthesis of these compounds remains highly desirable. Thus, the present work developed the synthesis of β-amino selenides with only 20 minutes of reaction time, following the conditions previously mentioned.