Safe and Efficient Process for the Preparation of Carmustine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cancer Drug Pharmacology Table
CANCER DRUG PHARMACOLOGY TABLE Cytotoxic Chemotherapy Drugs are classified according to the BC Cancer Drug Manual Monographs, unless otherwise specified (see asterisks). Subclassifications are in brackets where applicable. Alkylating Agents have reactive groups (usually alkyl) that attach to Antimetabolites are structural analogues of naturally occurring molecules DNA or RNA, leading to interruption in synthesis of DNA, RNA, or required for DNA and RNA synthesis. When substituted for the natural body proteins. substances, they disrupt DNA and RNA synthesis. bendamustine (nitrogen mustard) azacitidine (pyrimidine analogue) busulfan (alkyl sulfonate) capecitabine (pyrimidine analogue) carboplatin (platinum) cladribine (adenosine analogue) carmustine (nitrosurea) cytarabine (pyrimidine analogue) chlorambucil (nitrogen mustard) fludarabine (purine analogue) cisplatin (platinum) fluorouracil (pyrimidine analogue) cyclophosphamide (nitrogen mustard) gemcitabine (pyrimidine analogue) dacarbazine (triazine) mercaptopurine (purine analogue) estramustine (nitrogen mustard with 17-beta-estradiol) methotrexate (folate analogue) hydroxyurea pralatrexate (folate analogue) ifosfamide (nitrogen mustard) pemetrexed (folate analogue) lomustine (nitrosurea) pentostatin (purine analogue) mechlorethamine (nitrogen mustard) raltitrexed (folate analogue) melphalan (nitrogen mustard) thioguanine (purine analogue) oxaliplatin (platinum) trifluridine-tipiracil (pyrimidine analogue/thymidine phosphorylase procarbazine (triazine) inhibitor) -

The Effect of High-Dose Thiotepa, Alone Or in Combination with Other
Bone Marrow Transplantation (2007) 40, 891–896 & 2007 Nature Publishing Group All rights reserved 0268-3369/07 $30.00 www.nature.com/bmt ORIGINAL ARTICLE The effect of high-dose thiotepa, alone or in combination with other chemotherapeutic agents, on a murine B-cell leukemia model simulating autologous stem cell transplantation A Abdul-Hai, L Weiss, D Ergas, IB Resnick, S Slavin and MY Shapira Department of Bone Marrow Transplantation and Cancer Immunotherapy, Hadassah–Hebrew University Medical Center, Jerusalem, Israel The use of thiotepa (TH) is increasing, especially in stem Introduction cell transplantation, mainly due to its safety and blood– brain barrier penetration. We evaluated the use of TH in a Thiotepa (TH, triethylene thiophosphoramide), an ethylene murine model simulating autologous stem cell transplan- amide, developed by Lederle Laboratories in 1952, tation, with or without additional agents. Between 1 and possesses mechlorethamine-like alkylating activity and 11 days following inoculation of BALB/c mice with 105– has been used clinically for over 35 years.1 It is of particular 108 B-cell leukemia (BCL1) cells (simulating pre-trans- use in breast cancer, mainly as second-line treatment.2 plant leukemia loads), each group received an ‘induction- TH has been given intrathecally for carcinomatous like’ irradiation and/or cytotoxic regimen. Animals were meningitis, and intravesically for bladder carcinoma.3,4 either followed without treatment, or an adoptive transfer Mild-to-moderate activity was observed in several solid (AT) was performed to untreated BALB/c mice. Admi- tumors and hematological malignancies.1,5 There has nistered alone without AT, high-dose TH did not change been, however, a sustained interest in defining clinical roles the time to appearance of leukemia. -

SUMMARY of PARTICULARLY HAZARDOUS SUBSTANCES (By
SUMMARY OF PARTICULARLY HAZARDOUS SUBSTANCES (by alpha) Key: SC -- Select Carcinogens RT -- Reproductive Toxins AT -- Acute Toxins SA -- Readily Absorbed Through the Skin DHS -- Chemicals of Interest Revised: 11/2012 ________________________________________________________ ___________ _ _ _ _ _ _ _ _ _ _ _ ||| | | | CHEMICAL NAME CAS # |SC|RT| AT | SA |DHS| ________________________________________________________ ___________ | _ | _ | _ | _ | __ | | | | | | | 2,4,5-T 000093-76-5 | | x | | x | | ABRIN 001393-62-0 | | | x | | | ACETALDEHYDE 000075-07-0 | x | | | | | ACETAMIDE 000060-35-5 | x | | | | | ACETOHYDROXAMIC ACID 000546-88-3 ||x| | x | | ACETONE CYANOHYDRIN, STABILIZED 000075-86-5 | | | x | | x | ACETYLAMINOFLUORENE,2- 000053-96-3 | x | | | | | ACID MIST, STRONG INORGANIC 000000-00-0 | x | | | | | ACROLEIN 000107-02-8 | | x | x | x | | ACRYLAMIDE 000079-06-1 | x | x | | x | | ACRYLONITRILE 000107-13-1 | x | x | x | x | | ACTINOMYCIN D 000050-76-0 ||x| | x | | ADIPONITRILE 000111-69-3 | | | x | | | ADRIAMYCIN 023214-92-8 | x | | | | | AFLATOXIN B1 001162-65-8 | x | | | | | AFLATOXIN M1 006795-23-9 | x | | | | | AFLATOXINS 001402-68-2 | x | | x | | | ALL-TRANS RETINOIC ACID 000302-79-4 | | x | | x | | ALPRAZOMAN 028981-97-7 | | x | | x | | ALUMINUM PHOSPHIDE 020859-73-8 | | | x | | x | AMANTADINE HYDROCHLORIDE 000665-66-7 | | x | | x | | AMINO-2,4-DIBROMOANTHRAQUINONE 000081-49-2 | x | | | | | AMINO-2-METHYLANTHRAQUINONE, 1- 000082-28-0 | x | | | | | AMINO-3,4-DIMETHYL-3h-IMIDAZO(4,5f)QUINOLINE,2- 077094-11-2 | x | | | | | AMINO-3,8-DIMETHYL-3H-IMIDAZO(4,5-f)QUINOXALINE, -
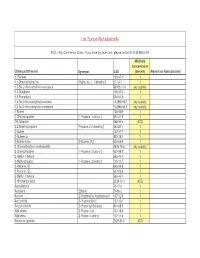
Homeland Security List
List Sorted Alphabetically ACG = Any Commercial Grade. If you have any questions, please contact EHS @ 898-5126. Minimum Concentration Chemical Of Interest Synonym CAS (percent) Amount on Hand (pounds) 1- Pentene 109-67-1 1 1,1-Dimethylhydrazine [Hydrazine, 1, 1-dimethyl-] 57-14-7 1 1,3-Bis(2-chloroethylthio)-n-propane 63905-10-2 any quantity 1,3-Butadiene 106-99-0 1 1,3-Pentadiene 504-60-9 1 1,4-Bis(2-chloroethylthio)-n-butane 142868-93-7 any quantity 1,5-Bis(2-chloroethylthio)-n-pentane 142868-94-8 any quantity 1-Butene 106-98-9 1 1-Chloropropylene [1-Propene, 1-chloro-] 590-21-6 1 1H-Tetrazole 288-94-8 ACG 2,2-Dimethylpropane [Propane, 2,2-dimethyl-] 463-82-1 1 2-Butene 107-01-7 1 2-Butene-cis 590-18-1 1 2-Butene-trans [2-Butene, (E)] 624-64-6 1 2-Chloroethylchloro-methylsulfide 2625-76-5 any quantity 2-Chloropropylene [1-Propene, 2-chloro-] 557-98-2 1 2-Methyl-1-butene 563-46-2 1 2-Methylpropene [1-Propene, 2-methyl-] 115-11-7 1 2-Pentene, (E)- 646-04-8 1 2-Pentene, (Z)- 627-20-3 1 3-Methyl-1-butene 563-45-1 1 5-Nitrobenzotriazol 2338-12-7 ACG Acetaldehyde 75-07-0 1 Acetylene [Ethyne] 74-86-2 1 Acrolein [2-Propenal] or Acrylaldehyde 107-02-8 1 Acrylonitrile [2-Propenenitrile] 107-13-1 1 Acrylyl chloride [2-Propenoyl chloride] 814-68-6 1 Allyl alcohol [2-Propen-1-ol] 107-18-6 1 Allylamine [2-Propen-1-amine] 107-11-9 1 Aluminum (powder) 7429-90-5 ACG Ammonia (anhydrous) 7664-41-7 1 Ammonia (conc. -
![Synthetic Approaches to Heterocyclic Bicyclo[2.1.0]Pentanes](https://docslib.b-cdn.net/cover/7157/synthetic-approaches-to-heterocyclic-bicyclo-2-1-0-pentanes-2177157.webp)
Synthetic Approaches to Heterocyclic Bicyclo[2.1.0]Pentanes
SYNTHETIC APPROACHES TO HETEROCYCLIC BICYCLO[2.1.0]PENTANES Rabah N. Alsulami A THESIS Submitted to the Graduate College of Bowling Green State University in partial fulfillment of The requirements for the degree of MASTER OF SCIENCE August 2011 Committee: Thomas H. Kinstle (Advisor) Marshall Wilson Alexander N. Tarnovsky ABSTRACT Thomas H. Kinstle, Advisor Bicyclic systems such as bicyclo[2.1.0]pentanes and 5-oxabicyclo[2.1.0]pentanes are known to display a variety of unique chemical properties associated with their high strain energy. To the best of our knowledge, there were no reports regarding synthesis and investigation of 5- azabicyclo[2.1.0]pentanes. Therefore, the initial goal of this research was synthesis of 5-azabicyclo[2.1.0]pentane and investigation of its chemical properties. The cycloaddition reaction of azides (58, 59, 61) to olefins (54, 55) with further elimination of nitrogen was chosen as a synthetic method in order to obtain the compounds of interest. Starting olefins (3,3-dimethyl-1-cyclobutene-1-carboxylic acid (54) and methyl 3,3-dimethyl-1-cyclobutene-1-carboxylate (55) and azides phenyl azide (58), p- toluenesulfonyl azide (59), and picryl azide (61) were successfully synthesized and characterized by NMR spectroscopy and GCMS spectrometry. The addition reaction between azides and olefins was performed under various conditions, such as different solvents and temperature; however, according to NMR spectroscopy and GCMS spectrometry, olefins (54, 55) do not undergo cycloaddition reaction with azides (58, 59, 61). In order to investigate that behavior, cycloaddition reactions of more reactive olefins (66, 68) with azides (58, 59, 61) were performed under a variety of conditions. -

Compendium Method TO-15
EPA/625/R-96/010b Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air Second Edition Compendium Method TO-15 Determination Of Volatile Organic Compounds (VOCs) In Air Collected In Specially-Prepared Canisters And Analyzed By Gas Chromatography/ Mass Spectrometry (GC/MS) Center for Environmental Research Information Office of Research and Development U.S. Environmental Protection Agency Cincinnati, OH 45268 January 1999 Method TO-15 Acknowledgements This Method was prepared for publication in the Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air, Second Edition (EPA/625/R-96/010b), which was prepared under Contract No. 68-C3-0315, WA No. 3-10, by Midwest Research Institute (MRI), as a subcontractor to Eastern Research Group, Inc. (ERG), and under the sponsorship of the U.S. Environmental Protection Agency (EPA). Justice A. Manning, John O. Burckle, and Scott Hedges, Center for Environmental Research Information (CERI), and Frank F. McElroy, National Exposure Research Laboratory (NERL), all in the EPA Office of Research and Development, were responsible for overseeing the preparation of this method. Additional support was provided by other members of the Compendia Workgroup, which include: • John O. Burckle, EPA, ORD, Cincinnati, OH • James L. Cheney, Corps of Engineers, Omaha, NB • Michael Davis, U.S. EPA, Region 7, KC, KS • Joseph B. Elkins Jr., U.S. EPA, OAQPS, RTP, NC • Robert G. Lewis, U.S. EPA, NERL, RTP, NC • Justice A. Manning, U.S. EPA, ORD, Cincinnati, OH • William A. McClenny, U.S. EPA, NERL, RTP, NC • Frank F. McElroy, U.S. EPA, NERL, RTP, NC • Heidi Schultz, ERG, Lexington, MA • William T. -

Anionic Polymerisation of Aziridines
MAX-PLANCK-INSTITUT FÜR POLYMERFORSCHUNG JOHANNES GUTENBERG‐UNIVERSITÄT MAINZ Anionic Polymerisation of Aziridines Diplomarbeit zur Erlangung des Grades eines Diplom‐Chemikers am Institut für Organische Chemie des Fachbereiches Chemie, Pharmazie und Geowissenschaften der Johannes Gutenberg-Universität Mainz vorgelegt von Laura Thomi geboren in Frankfurt am Main Mainz 2013 Diese Arbeit wurde in der Zeit von November 2012 bis Juli 2013 am Institut für Organische Chemie der Johannes Gutenberg‐Universität Mainz und am Max-Planck-Institut für Polymerforschung in Mainz unter der Betreuung von Herrn Prof. Dr. Holger Frey und Frau Prof. Dr. Katharina Landfester durchgeführt. für meine Eltern “We live on an island surrounded by a sea of ignorance. As our island of knowledge grows, so does the shore of our ignorance.” - John Archibald Wheeler Danksagung Mein Dank gilt Herrn Prof. Dr. Holger Frey und Frau Prof. Dr. Katharina Landfester für die Bereitstellung des Themas und die ausgezeichneten Arbeitsbedingungen. Zudem danke ich Herrn Dr. Frederik Wurm für die freundliche Aufnahme in seine Gruppe und die hervorragende Betreuung der Arbeit. Bei allen Mitgliedern der Arbeitsgruppe Frey bedanke ich mich für die wunderbare Arbeitsatmosphäre und die Unterstützung bei dieser Arbeit. Herrn Christian Moers und Herrn Jan Seiwert danke ich, dass sie mir die anionische Polymerisation von Styrol und Ethylenoxid näher gebracht haben. Bei Frau Anna Hesse und Herrn Christian Moers bedanke ich mich für das Korrekturlesen dieser Arbeit. Frau Katja Weber danke ich für die Bereitstellung des 2-Methyl-N-tosylaziridins. Für die GPC Messungen danke ich Frau Christine Rosenauer und insbesondere Frau Monika Schmelzer. Bei Frau Dr. Elena Berger-Nicoletti möchte ich mich für zahlreiche MALDI Messungen bedanken. -

Synthesis and Biological Activity of N-Acyl Aziridines a Dissertation
Synthesis and Biological Activity of N-Acyl Aziridines A dissertation presented to the faculty of the College of Arts and Sciences of Ohio University In partial fulfillment of the requirements for the degree Doctor of Philosophy Greggory M. Wells April 2016 © 2016 Greggory M. Wells. All Rights Reserved. 2 This dissertation titled Synthesis and Biological Activity of N-Acyl Aziridines by GREGGORY M. WELLS has been approved for the Department of Chemistry and Biochemistry and the College of Arts and Sciences by Stephen C. Bergmeier Professor of Chemistry and Biochemistry Robert Frank Dean, College of Arts and Sciences 3 ABSTRACT WELLS, GREGGORY M., Ph.D., April 2016, Chemistry Synthesis and Biological Activity of N-Acyl Aziridines Director of Dissertation: Stephen C. Bergmeier The development of new antimicrobial drugs is essential as the human population continues to build resistance to current treatments. Peptidomimetic compounds, those synthesized to mimic the behavior of naturally occurring biological proteins, have demonstrated promise in this area. Using bicyclic aziridine ring opening reactions, a library of N-acyl aziridinyl peptide isosteres has been synthesized and submitted to biological assays for cysteine protease inhibition, a common pathway to suppression of bacteria. Most of the compounds tested showed good activity against cathepsin B. This research required a novel synthetic approach to selectively generating oxazolidinones and aziridinyl ureas from fused bicyclic aziridines, which was accomplished with solvent selection, nucleophilic amine stoichiometry, and aziridine substitution. To extend the peptidic nature of these compounds, some success was achieved with acylation and amide coupling reactions. A practical approach to generating enantiomerically pure bicyclic aziridines was investigated, however the best enantioselective conditions provided only a 3 : 1 ratio. -

The Chemistry of Heterocycles Structure, Reactions, Syntheses
The Chemistry of Heterocycles Structure, Reactions, Syntheses, and Applications Mohammad Jafarzadeh Faculty of Chemistry, Razi University The Chemistry of Heterocycles, (Second Edition). By Theophil Eicher and Siegfried Hauptmann, Wiley-VCH Veriag GmbH, 2003 1 24 3 Three-Membered Heterocycles 3.2 Thiirane 24 3 Three-Membered Heterocycles 3.2 Thiirane Thiiranes are also known as episulfides. As a result of the greater atomic radius of the S-atom, the three atoms form an acute-angled triangle (see Fig. 3.2). [A] Thiiranes are also known as episulfides. As a result of the greater atomic radius of the S-atom, the 3.2 Thiirane three atoms form an acute-angled triangle. Fig. 3.2 Structure of thiirane (bond lengths in pm, bond angles in degrees) The thermochemically determined strainThenthalpye thermochemicallof thiiraney determineof 83 kJd molstrai-1n isenthalplessythan of thiiranthateof ofoxirane 83 kJ mo. H is less than that of oxirane. The ionization potential amounts to 9.05TheV,e ionizatiothe dipolen potentiamomentl amountto 1.66s toD 9.0. Both5 eVvalues, the dipolaree belowmomenthoset to 1.66 D. Both values are below Thiiranes are also known aofsoxirane episulfides. The chemical. Ashiftss a resulin the NMRt thosofspectrae thof oxiranee greateare. �ThH e= chemica2r. 27atomi, �l Cshift= 18sc i .n1radiu .the NMRs spectrof ath are S ^S-atom = 2.27, Sc ,=18.1. the three atoms form an acute-angled triangle (see Fig. 3.2)Th.e properties of the thiiranes are primarily due to ring strain. In spite of the smaller strain en- [B] The properties of the thiiranes are primarilythalpydue, tothiiranringestrain is thermall. -

Unlocking the Synthetic Potential of Aziridine and Cyclopropane-Fused Quinolin-2-Ones by Regioselective Fragmentation of Its Three-Membered Rings
Arabian Journal of Chemistry (2020) 13, 2702–2714 King Saud University Arabian Journal of Chemistry www.ksu.edu.sa www.sciencedirect.com ORIGINAL ARTICLE Unlocking the synthetic potential of aziridine and cyclopropane-fused quinolin-2-ones by regioselective fragmentation of its three-membered rings Javier Diaz a, Daniel Rodenas a, Francisco-Jose Ballester a, Mateo Alajarin a, Raul-Angel Orenes b, Pilar Sanchez-Andrada a,*, Angel Vidal a,* a Departamento de Quı´mica Orga´nica, Universidad de Murcia, Facultad de Quı´mica, Regional Campus of International Excellence ‘‘Campus Mare Nostrum”, Espinardo, 30100 Murcia, Spain b Servicio Universitario de Instrumentacio´n Cientı´fica, Universidad de Murcia, Regional Campus of International Excellence ‘‘Campus Mare Nostrum”, Espinardo, 30100 Murcia, Spain Received 26 March 2018; accepted 2 July 2018 Available online 11 July 2018 KEYWORDS Abstract The cyclization of cis-2-(2-azidophenyl)-1-benzyl-3-ethoxycarbonylaziridines and trans- Aziridino[2,3-c]quinolin- 2-(2-azidophenyl)-3-nitrocyclopropane-1,1-dicarboxylates yielded the respective aziridino[2,3-c] 2-ones; quinolin-2-ones and cyclopropa[c]quinolin-2-ones. Ring-opening of the aziridine-fused species Cyclopropa[c]quinolin- under silica gel catalysis provided 3-aminoquinolin-2-ones whereas the ring-expansion of the 2-ones; cyclopropane-fused derivatives by the action of sodium hydride gave 1-benzazepin-2-ones, in both 3-aminoquinolin-2-ones; cases in a regioselective manner. A computational study using DFT methods revealed that the Benzazepin-2-ones; mechanism for the transformation of cyclopropa[c]quinolin-2-ones into 1-benzazepin-2-ones p 6 -electrocyclic ring opening; involves the initial deprotonation step of its amide function followed by two pericyclic events: a [1,5]-H shift 6p-electrocyclic ring opening and a subsequent [1,5]-H shift. -

Recent Synthesis of Thietanes
Recent synthesis of thietanes Jiaxi Xu Review Open Access Address: Beilstein J. Org. Chem. 2020, 16, 1357–1410. State Key Laboratory of Chemical Resource Engineering, Department doi:10.3762/bjoc.16.116 of Organic Chemistry, College of Chemistry, Beijing University of Chemical Technology, Beijing 100029, People’s Republic of China Received: 29 March 2020 Accepted: 26 May 2020 Email: Published: 22 June 2020 Jiaxi Xu - [email protected] Associate Editor: B. Nay Keywords: © 2020 Xu; licensee Beilstein-Institut. cycloaddition; cyclization; ring contraction; ring expansion; thietane; License and terms: see end of document. thiotherification Abstract Thietanes are important aliphatic four-membered thiaheterocycles that are found in the pharmaceutical core and structural motifs of some biological compounds. They are also useful intermediates in organic synthesis. Various synthetic methods of thietanes have been developed, including inter- and intramolecular nucleophilic thioetherifications, photochemical [2 + 2] cycloadditions, ring expansions and contractions, nucleophilic cyclizations, and some miscellaneous methods. The recently developed methods provide some new strategies for the efficient preparation of thietanes and their derivatives. This review focuses on the synthetic methods to construct thietane backbones developed during 1966 to 2019. Review 1. Introduction Thietanes are a class of important aliphatic four-membered thia- acyclic and heterocyclic compounds [10,11]. Several synthetic heterocycles. Some simple alkyl and dialkyl thietanes -

Department of Homeland Security
Appendix A Department of Homeland Security Chemicals of Interest Appendix A Chemical Of Interest Synonym CAS Acetaldehyde 75-07-0 Acetone cyanohydrin, stabilized 75-86-5 Acetyl bromide 506-96-7 Acetyl chloride 75-36-5 Acetyl iodide 507-02-8 Acetylene [Ethyne] 74-86-2 Acrolein [2-Propenal] or Acrylaldehyde 107-02-8 Acrylonitrile [2-Propenenitrile] 107-13-1 Acrylyl chloride [2-Propenoyl chloride] 814-68-6 Allyl alcohol [2-Propen-1-ol] 107-18-6 Allylamine [2-Propen-1-amine] 107-11-9 Allyltrichlorosilane, stabilized 107-37-9 Aluminum (powder) 7429-90-5 Aluminum bromide, anhydrous 7727-15-3 Aluminum chloride, anhydrous 7446-70-0 Aluminum phosphide 20859-73-8 Ammonia (anhydrous) 7664-41-7 Ammonia (conc. 20% or greater) 7664-41-7 Ammonium nitrate, [with more than 0.2 percent combustible substances, including any organic 6484-52-2 substance calculated as carbon, to the exclusion of any other added substance] Ammonium nitrate, solid [nitrogen concentration of 23% nitrogen or 6484-52-2 greater] Ammonium perchlorate 7790-98-9 Ammonium picrate 131-74-8 Amyltrichlorosilane 107-72-2 Antimony pentafluoride 7783-70-2 Arsenic trichloride [Arsenous trichloride] 7784-34-1 Arsine 7784-42-1 Barium azide 18810-58-7 1,4-Bis(2-chloroethylthio)-n- 142868-93-7 butane Bis(2-chloroethylthio)methane 63869-13-6 Bis(2-chloroethylthiomethyl)ether 63918-90-1 1,5-Bis(2-chloroethylthio)-n- 142868-94-8 pentane 1,3-Bis(2-chloroethylthio)-n- 63905-10-2 propane Boron tribromide 10294-33-4 Boron trichloride [Borane, trichloro] 10294-34-5 Boron trifluoride [Borane, trifluoro]