Pp-03-25-New Dots.Qxd 10/23/02 2:41 PM Page 657
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United States Patent Office Patented Sept
3,149,913 United States Patent Office Patented Sept. 22, 1964 2 may vary over a wide range and may be as little as 1% 3,49,913 and as much as 50% and even higher. Particularly ad PROCESS FOR PRODUCING NETROSYL vantageous is the use of nitric acid in amounts such that SULFURECACE) d the resulting nitrosylsulfuric acid concentration approxi ALouis L. Ferstandig, El Cerrito, and Paul C. Condit, San 5 mates saturation values in order to afford maximum pro Asseino,poration, Calif.,San Francisco,assignors toCalif., California a corporation Research Cor of duction per unit reactor volume and yet avoid deposition Beavy are of solids. The solubility of nitrosylsulfuric acid in ap No Drawing. Fied June 14, 1961, Ser. No. 116,957 proximately 100% sulfuric acid ranges from about 48 3 (Caims. (CE. 23-39) grams per 100 grams of solution at 0° C. up to about 68 grams at 50 C. with, of course, a lesser solubility below This invention relates to a proces for the production of O 0 C. and a greater above 50° C. Although the presence nitrosylsulfuric acid. of the precipitated nitrosylsulfuric acid is usually a source Nitrosyisulfuric acid is particularly desirable for use of mechanical inconvenience, advantage may be taken of in the production of caprolactam from hexahydrobenzoic it by removal of the solid nitrosylsulfuric acid by filtra acid. Nitrosylsulfuric acid has long been known as an 5 tion and subsequent recycle of the mother liquor to the intermediate in connection with the lead-chamber sulfuric reaction Zone. acid process in which it is converted to Sulfuric acid with in general, the effective temperature range of the process concurrent liberation of nitric oxide in a reaction with is defined by the requirement that the reaction medium be sulfur dioxide. -

New Synthesis Routes for Production of Ε-Caprolactam by Beckmann
New synthesis routes for production of ε-caprolactam by Beckmann rearrangement of cyclohexanone oxime and ammoximation of cyclohexanone over different metal incorporated molecular sieves and oxide catalysts Von der Fakultät für Mathematik, Informatik und Naturwissenschaften der RWTH Aachen University zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften genehmigte Dissertation vorgelegt von Anilkumar Mettu aus Guntur/Indien Berichter: Universitätprofessor Dr. Wolfgang F. Hölderich Universitätprofessor Dr. Carsten Bolm Tag der mündlichen Prüfung: 29.01.2009 Diese Dissertation ist auf den Internetseiten der Hochschulbibliothek online verfügbar. Dedicated to my Parents This work reported here has been carried out at the Institute for Chemical Technolgy and Heterogeneous Catalysis der Fakultät für Mathematik, Informatik und Naturwissenschaften in the University of Technology, RWTH Aachen under supervision of Prof. Dr. Wolfgang F. Hölderich between June 2005 and August 2008. ACKNOWLEDGEMENTS I would like to express my deepest sence of gratitude to my supervisor Prof. Dr. rer. nat. W. F. Hölderich for giving me the opportunity to do my doctoral study in his group. His guidance and teaching classes have allowed me to grow and learn my subject during my Ph.d. He has provided many opportunities for me to increase my abilities as a researcher and responsibilities as a team member. I am grateful for the financial support of this work from Sumitomo Chemicals Co., Ltd, Niihama, Japan (Part One) and Uhde Inventa-Fischer GmBH, Berlin (Part Two). Our collaborators at Sumitomo Chemicals Co., Ltd (Dr. C. Stoecker) and Uhde Inventa- Fischer GmBH (Dr. R. Schaller and Dr. A. Pawelski) provided thoughtful guidance and suggestions for each project. -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Cancer Drug Pharmacology Table
CANCER DRUG PHARMACOLOGY TABLE Cytotoxic Chemotherapy Drugs are classified according to the BC Cancer Drug Manual Monographs, unless otherwise specified (see asterisks). Subclassifications are in brackets where applicable. Alkylating Agents have reactive groups (usually alkyl) that attach to Antimetabolites are structural analogues of naturally occurring molecules DNA or RNA, leading to interruption in synthesis of DNA, RNA, or required for DNA and RNA synthesis. When substituted for the natural body proteins. substances, they disrupt DNA and RNA synthesis. bendamustine (nitrogen mustard) azacitidine (pyrimidine analogue) busulfan (alkyl sulfonate) capecitabine (pyrimidine analogue) carboplatin (platinum) cladribine (adenosine analogue) carmustine (nitrosurea) cytarabine (pyrimidine analogue) chlorambucil (nitrogen mustard) fludarabine (purine analogue) cisplatin (platinum) fluorouracil (pyrimidine analogue) cyclophosphamide (nitrogen mustard) gemcitabine (pyrimidine analogue) dacarbazine (triazine) mercaptopurine (purine analogue) estramustine (nitrogen mustard with 17-beta-estradiol) methotrexate (folate analogue) hydroxyurea pralatrexate (folate analogue) ifosfamide (nitrogen mustard) pemetrexed (folate analogue) lomustine (nitrosurea) pentostatin (purine analogue) mechlorethamine (nitrogen mustard) raltitrexed (folate analogue) melphalan (nitrogen mustard) thioguanine (purine analogue) oxaliplatin (platinum) trifluridine-tipiracil (pyrimidine analogue/thymidine phosphorylase procarbazine (triazine) inhibitor) -

Download Safety Data Sheet (SDS)
SAFETY DATA SHEET 1. Identification Product identifier Strike 80 Soil Fumigant Other means of identification SDS number 180-AUS-TCA Recommended use Soil fumigant NOTE TO PESTICIDE HANDLERS: If the pesticide product end-use labeling contains hazard information, specific instructions, or requirements that conflict with this Safety Data Sheet (SDS), follow the hazard information, instructions, or requirements on the labeling. Restrictions on use Use of this product requires supervision by a qualified pesticide applicator. Details of manufacturer or importer TriCal Australia Pty Ltd Address 5 Chamberlain Street, Wingfield, SA 5013, Australia Telephone 08 8347 3838 E-mail [email protected] Emergency phone number CHEMTREC (Australia) 02 9037 2994 (24/7) POISONS INFORMATION CENTRE 13 11 26 2. Hazard(s) identification Physical hazards Not classified. Health hazards Acute toxicity, oral Category 2 Acute toxicity, dermal Category 2 Acute toxicity, inhalation Category 1 Skin corrosion/irritation Category 1C Serious eye damage/eye irritation Category 1 Sensitization, skin Category 1 Carcinogenicity Category 2 Specific target organ toxicity, Category 1 (respiratory system damage) single exposure Specific target organ toxicity, Category 3 (respiratory tract irritation) single exposure Specific target organ toxicity, Category 1 (respiratory tract/lungs) repeated exposure Environmental hazards Hazardous to the aquatic environment, Category 1 acute hazard Hazardous to the aquatic environment, Category 2 long-term hazard Label elements Skull Corrosion Health Exclamation Environment Signal word DANGER Hazard statement Fatal if swallowed, in contact with skin or if inhaled. May cause an allergic skin reaction. Causes serious eye damage. Causes severe skin burns and eye damage. Strike 80 Soil Fumigant November 25, 2019 Page 1 of 11 May cause respiratory irritation. -

Aug. 29, 1967 Yoshi KAZU ITO ETAL 3,338,887 PREPARATION of NITROSYL CHLORIDE Filed Dec
Aug. 29, 1967 Yoshi KAZU ITO ETAL 3,338,887 PREPARATION OF NITROSYL CHLORIDE Filed Dec. 26, 1962 INVENTORS YOSHIKAZU ITO FUMO NS-KAWA 2%-42.TAKAO WAMURA ATTORNEY 3,338,887 United States Patent Office Patented Aug. 29, 1967 1. 2 3,338,887 be taken out of the cyclic system in order to maintain PREPARATION OF NITROSYL CHLORIDE its material balance, or, alternatively, the water has to Yoshikazu to, Mizuho-ku, Nagoya, and Fumio Nishi be taken out of the cyclic system by distillation of the kawa and Takao Iwamura, Minami-ku, Nagoya, Japan, spent liquor under reduced pressure. assignors to Toyo Rayon Kabushiki Kaisha, Tokyo, Japan, a corporation of Japan While the spent liquor which has been taken out to Filed Dec. 26, 1962, Ser. No. 246,914 the outside of the system by the above method can be Claims priority, application Japan, Dec. 26, 1961, used for other purposes as sulfuric acid by being con 36/46,743 verted thereto by the conventional nitric oxide process 1 Claim. (Cl. 260-239.3) for sulfuric acid manufacture, the recovery of the oxides 10 of nitrogen which are formed in this instance is a dis This invention relates to a method of preparing nitro advantage from the commercial standpoint. Furthermore, syl chloride which makes it possible to prepare nitrosyl since a small amount of hydrochloric acid is contained chloride cyclically with advantage and effectiveness on in the cycling spent liquor, hydrochloric acid is also a commercial scale, and in which the liquid portion which contained in the sulfuric acid formed. -

The Effect of High-Dose Thiotepa, Alone Or in Combination with Other
Bone Marrow Transplantation (2007) 40, 891–896 & 2007 Nature Publishing Group All rights reserved 0268-3369/07 $30.00 www.nature.com/bmt ORIGINAL ARTICLE The effect of high-dose thiotepa, alone or in combination with other chemotherapeutic agents, on a murine B-cell leukemia model simulating autologous stem cell transplantation A Abdul-Hai, L Weiss, D Ergas, IB Resnick, S Slavin and MY Shapira Department of Bone Marrow Transplantation and Cancer Immunotherapy, Hadassah–Hebrew University Medical Center, Jerusalem, Israel The use of thiotepa (TH) is increasing, especially in stem Introduction cell transplantation, mainly due to its safety and blood– brain barrier penetration. We evaluated the use of TH in a Thiotepa (TH, triethylene thiophosphoramide), an ethylene murine model simulating autologous stem cell transplan- amide, developed by Lederle Laboratories in 1952, tation, with or without additional agents. Between 1 and possesses mechlorethamine-like alkylating activity and 11 days following inoculation of BALB/c mice with 105– has been used clinically for over 35 years.1 It is of particular 108 B-cell leukemia (BCL1) cells (simulating pre-trans- use in breast cancer, mainly as second-line treatment.2 plant leukemia loads), each group received an ‘induction- TH has been given intrathecally for carcinomatous like’ irradiation and/or cytotoxic regimen. Animals were meningitis, and intravesically for bladder carcinoma.3,4 either followed without treatment, or an adoptive transfer Mild-to-moderate activity was observed in several solid (AT) was performed to untreated BALB/c mice. Admi- tumors and hematological malignancies.1,5 There has nistered alone without AT, high-dose TH did not change been, however, a sustained interest in defining clinical roles the time to appearance of leukemia. -

SUMMARY of PARTICULARLY HAZARDOUS SUBSTANCES (By
SUMMARY OF PARTICULARLY HAZARDOUS SUBSTANCES (by alpha) Key: SC -- Select Carcinogens RT -- Reproductive Toxins AT -- Acute Toxins SA -- Readily Absorbed Through the Skin DHS -- Chemicals of Interest Revised: 11/2012 ________________________________________________________ ___________ _ _ _ _ _ _ _ _ _ _ _ ||| | | | CHEMICAL NAME CAS # |SC|RT| AT | SA |DHS| ________________________________________________________ ___________ | _ | _ | _ | _ | __ | | | | | | | 2,4,5-T 000093-76-5 | | x | | x | | ABRIN 001393-62-0 | | | x | | | ACETALDEHYDE 000075-07-0 | x | | | | | ACETAMIDE 000060-35-5 | x | | | | | ACETOHYDROXAMIC ACID 000546-88-3 ||x| | x | | ACETONE CYANOHYDRIN, STABILIZED 000075-86-5 | | | x | | x | ACETYLAMINOFLUORENE,2- 000053-96-3 | x | | | | | ACID MIST, STRONG INORGANIC 000000-00-0 | x | | | | | ACROLEIN 000107-02-8 | | x | x | x | | ACRYLAMIDE 000079-06-1 | x | x | | x | | ACRYLONITRILE 000107-13-1 | x | x | x | x | | ACTINOMYCIN D 000050-76-0 ||x| | x | | ADIPONITRILE 000111-69-3 | | | x | | | ADRIAMYCIN 023214-92-8 | x | | | | | AFLATOXIN B1 001162-65-8 | x | | | | | AFLATOXIN M1 006795-23-9 | x | | | | | AFLATOXINS 001402-68-2 | x | | x | | | ALL-TRANS RETINOIC ACID 000302-79-4 | | x | | x | | ALPRAZOMAN 028981-97-7 | | x | | x | | ALUMINUM PHOSPHIDE 020859-73-8 | | | x | | x | AMANTADINE HYDROCHLORIDE 000665-66-7 | | x | | x | | AMINO-2,4-DIBROMOANTHRAQUINONE 000081-49-2 | x | | | | | AMINO-2-METHYLANTHRAQUINONE, 1- 000082-28-0 | x | | | | | AMINO-3,4-DIMETHYL-3h-IMIDAZO(4,5f)QUINOLINE,2- 077094-11-2 | x | | | | | AMINO-3,8-DIMETHYL-3H-IMIDAZO(4,5-f)QUINOXALINE, -
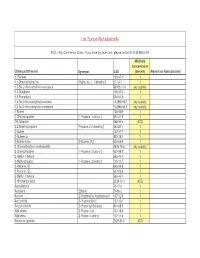
Homeland Security List
List Sorted Alphabetically ACG = Any Commercial Grade. If you have any questions, please contact EHS @ 898-5126. Minimum Concentration Chemical Of Interest Synonym CAS (percent) Amount on Hand (pounds) 1- Pentene 109-67-1 1 1,1-Dimethylhydrazine [Hydrazine, 1, 1-dimethyl-] 57-14-7 1 1,3-Bis(2-chloroethylthio)-n-propane 63905-10-2 any quantity 1,3-Butadiene 106-99-0 1 1,3-Pentadiene 504-60-9 1 1,4-Bis(2-chloroethylthio)-n-butane 142868-93-7 any quantity 1,5-Bis(2-chloroethylthio)-n-pentane 142868-94-8 any quantity 1-Butene 106-98-9 1 1-Chloropropylene [1-Propene, 1-chloro-] 590-21-6 1 1H-Tetrazole 288-94-8 ACG 2,2-Dimethylpropane [Propane, 2,2-dimethyl-] 463-82-1 1 2-Butene 107-01-7 1 2-Butene-cis 590-18-1 1 2-Butene-trans [2-Butene, (E)] 624-64-6 1 2-Chloroethylchloro-methylsulfide 2625-76-5 any quantity 2-Chloropropylene [1-Propene, 2-chloro-] 557-98-2 1 2-Methyl-1-butene 563-46-2 1 2-Methylpropene [1-Propene, 2-methyl-] 115-11-7 1 2-Pentene, (E)- 646-04-8 1 2-Pentene, (Z)- 627-20-3 1 3-Methyl-1-butene 563-45-1 1 5-Nitrobenzotriazol 2338-12-7 ACG Acetaldehyde 75-07-0 1 Acetylene [Ethyne] 74-86-2 1 Acrolein [2-Propenal] or Acrylaldehyde 107-02-8 1 Acrylonitrile [2-Propenenitrile] 107-13-1 1 Acrylyl chloride [2-Propenoyl chloride] 814-68-6 1 Allyl alcohol [2-Propen-1-ol] 107-18-6 1 Allylamine [2-Propen-1-amine] 107-11-9 1 Aluminum (powder) 7429-90-5 ACG Ammonia (anhydrous) 7664-41-7 1 Ammonia (conc. -

United States Patent (19) 11) 4,182,708 Landler Et Al
United States Patent (19) 11) 4,182,708 Landler et al. 45 Jan. 8, 1980 54 PROCESS FOR THE PREPARATION OF 58) Field of Search ............ 260/144 P, 208, 174-193, AZO PIGMENTS BY DAZOTIZING INA 260/157, 162, 163, 155, 203, 204; 106/308, 288 DIPOLARAPROTC ORGANIC SOLVENT AND AZO PIGMENTS OBTANED Q THEREFROM (56) References Cited U.S. PATENT DOCUMENTS 75 Inventors: Josef Landler, Hofheim; Klatis 2,683,708 2/1954 Dickey et al. ....................... 260/158 Htinger, Kelkheim; Erhard Wörfel, 2,790,791 4/1957 Towne et al......................... 260/158 Hattersheim, all of Fed. Rep. of 3,213,080 10/1965 Bloom et al. .... ... 260/155 Germany 3,382,228 5/1968 Ferrari et al. ........................ 260/158 3,642,769 2/1972 Moritz et al. ... 260/207 73) Assignee: Hoechst Aktiengesellschaft, 3,711,461 1/1973 Pretzer et al. ... 260/154 Frankfurt am Main, Fed. Rep. of 3,781,266 12/1973 Dietz et al. .. ... 260/157 Germany 3,793,305 2/1974 Balon ................................... 260/154 Primary Examiner-Floyd D. Higel (21) Appl. No.: 761,071 Attorney, Agent, or Firm-Curtis, Morris & Safford 57 ABSTRACT 22 Filed: Jan. 21, 1977 Azo pigments are obtained by diazotizing a diazotizable aromatic amine without solubilizing groups in anhy Related U.S. Application Data drous dipolar aprotic water-miscible solvents with the 63 Continuation-in-part of Ser. No. 619,460, Oct. 3, 1975, stoichiometric amount or a small excess of nitrosylsulfu abandoned, which is a continuation of Ser. No. ric acid or nitrosyl chloride, coupling the diazonium 325,549, Jan. 22, 1973, abandoned. -

Deepak Nitrite Limitd
Deepak Nitrite Ltd Technical Data Sheet Nitrosylsulphuric acid (NSA) ( Domestic ) 1. Introduction Nitrosylsulfuric acid is the chemical compound with the formula NOHSO4. It is a colourless solid that is used industrially in the production of caprolactam. 1. Product: Nitrosylsulphuric acid (NSA) 2. CAS No: 7782-78-7 3. Molecular Formula: HNO5S 4. Molecular Weight: 127.08 2. Physical Properties: Physical state: viscous liquid Appearance: Clear yellow to green viscous liquid Melting Point : -10 °C Stability: Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are opened must be carefully resealed and kept upright to prevent leakage. Handle and store under inert gas. Density 1.890 – 1.895 g/cm3 ( at 20 °C ) Boiling point :- Decomposes Solubility in water :- Decomposes Solubility :- Soluble in H2SO4 Effective Date : 21.11.2012 Page 1 of 4 Deepak Nitrite Ltd 3. Product Quality Specification: Sr Parameter Standard Grade Un coated Number 01 Physical Clear yellow to greenish viscous liquid Appearance 02 Nitrosylsulphuric 35.0 to 40.0 %w/w acid (NSA) 03 Acidity as H2SO4 54.0 %w/w Min. Our products are not meant for use as food or drug additives. 4. Packing Information: Sr. Grade Packing Secondary Packing Number 01 Liquor In Tanker DNL Can customize packing for different quantities. 5.PRODUCT USES Chemical Properties Yellowish viscous liquid Usage Nitrosylsulfuric acid is used for diazotisation, nitrosation, oxidation and oximation reactions. Product Data Sheet General Description Shipped in solution with sulfuric acid (solutions are usually 40% Nitrosylsulfuric acid and 54% sulfuric acid (Hawley)). Solution is a straw-colored oily liquid with a sharp odor. -
![Synthetic Approaches to Heterocyclic Bicyclo[2.1.0]Pentanes](https://docslib.b-cdn.net/cover/7157/synthetic-approaches-to-heterocyclic-bicyclo-2-1-0-pentanes-2177157.webp)
Synthetic Approaches to Heterocyclic Bicyclo[2.1.0]Pentanes
SYNTHETIC APPROACHES TO HETEROCYCLIC BICYCLO[2.1.0]PENTANES Rabah N. Alsulami A THESIS Submitted to the Graduate College of Bowling Green State University in partial fulfillment of The requirements for the degree of MASTER OF SCIENCE August 2011 Committee: Thomas H. Kinstle (Advisor) Marshall Wilson Alexander N. Tarnovsky ABSTRACT Thomas H. Kinstle, Advisor Bicyclic systems such as bicyclo[2.1.0]pentanes and 5-oxabicyclo[2.1.0]pentanes are known to display a variety of unique chemical properties associated with their high strain energy. To the best of our knowledge, there were no reports regarding synthesis and investigation of 5- azabicyclo[2.1.0]pentanes. Therefore, the initial goal of this research was synthesis of 5-azabicyclo[2.1.0]pentane and investigation of its chemical properties. The cycloaddition reaction of azides (58, 59, 61) to olefins (54, 55) with further elimination of nitrogen was chosen as a synthetic method in order to obtain the compounds of interest. Starting olefins (3,3-dimethyl-1-cyclobutene-1-carboxylic acid (54) and methyl 3,3-dimethyl-1-cyclobutene-1-carboxylate (55) and azides phenyl azide (58), p- toluenesulfonyl azide (59), and picryl azide (61) were successfully synthesized and characterized by NMR spectroscopy and GCMS spectrometry. The addition reaction between azides and olefins was performed under various conditions, such as different solvents and temperature; however, according to NMR spectroscopy and GCMS spectrometry, olefins (54, 55) do not undergo cycloaddition reaction with azides (58, 59, 61). In order to investigate that behavior, cycloaddition reactions of more reactive olefins (66, 68) with azides (58, 59, 61) were performed under a variety of conditions.