The Chemistry of Heterocycles Structure, Reactions, Syntheses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Cancer Drug Pharmacology Table
CANCER DRUG PHARMACOLOGY TABLE Cytotoxic Chemotherapy Drugs are classified according to the BC Cancer Drug Manual Monographs, unless otherwise specified (see asterisks). Subclassifications are in brackets where applicable. Alkylating Agents have reactive groups (usually alkyl) that attach to Antimetabolites are structural analogues of naturally occurring molecules DNA or RNA, leading to interruption in synthesis of DNA, RNA, or required for DNA and RNA synthesis. When substituted for the natural body proteins. substances, they disrupt DNA and RNA synthesis. bendamustine (nitrogen mustard) azacitidine (pyrimidine analogue) busulfan (alkyl sulfonate) capecitabine (pyrimidine analogue) carboplatin (platinum) cladribine (adenosine analogue) carmustine (nitrosurea) cytarabine (pyrimidine analogue) chlorambucil (nitrogen mustard) fludarabine (purine analogue) cisplatin (platinum) fluorouracil (pyrimidine analogue) cyclophosphamide (nitrogen mustard) gemcitabine (pyrimidine analogue) dacarbazine (triazine) mercaptopurine (purine analogue) estramustine (nitrogen mustard with 17-beta-estradiol) methotrexate (folate analogue) hydroxyurea pralatrexate (folate analogue) ifosfamide (nitrogen mustard) pemetrexed (folate analogue) lomustine (nitrosurea) pentostatin (purine analogue) mechlorethamine (nitrogen mustard) raltitrexed (folate analogue) melphalan (nitrogen mustard) thioguanine (purine analogue) oxaliplatin (platinum) trifluridine-tipiracil (pyrimidine analogue/thymidine phosphorylase procarbazine (triazine) inhibitor) -

Download Safety Data Sheet (SDS)
SAFETY DATA SHEET 1. Identification Product identifier Strike 80 Soil Fumigant Other means of identification SDS number 180-AUS-TCA Recommended use Soil fumigant NOTE TO PESTICIDE HANDLERS: If the pesticide product end-use labeling contains hazard information, specific instructions, or requirements that conflict with this Safety Data Sheet (SDS), follow the hazard information, instructions, or requirements on the labeling. Restrictions on use Use of this product requires supervision by a qualified pesticide applicator. Details of manufacturer or importer TriCal Australia Pty Ltd Address 5 Chamberlain Street, Wingfield, SA 5013, Australia Telephone 08 8347 3838 E-mail [email protected] Emergency phone number CHEMTREC (Australia) 02 9037 2994 (24/7) POISONS INFORMATION CENTRE 13 11 26 2. Hazard(s) identification Physical hazards Not classified. Health hazards Acute toxicity, oral Category 2 Acute toxicity, dermal Category 2 Acute toxicity, inhalation Category 1 Skin corrosion/irritation Category 1C Serious eye damage/eye irritation Category 1 Sensitization, skin Category 1 Carcinogenicity Category 2 Specific target organ toxicity, Category 1 (respiratory system damage) single exposure Specific target organ toxicity, Category 3 (respiratory tract irritation) single exposure Specific target organ toxicity, Category 1 (respiratory tract/lungs) repeated exposure Environmental hazards Hazardous to the aquatic environment, Category 1 acute hazard Hazardous to the aquatic environment, Category 2 long-term hazard Label elements Skull Corrosion Health Exclamation Environment Signal word DANGER Hazard statement Fatal if swallowed, in contact with skin or if inhaled. May cause an allergic skin reaction. Causes serious eye damage. Causes severe skin burns and eye damage. Strike 80 Soil Fumigant November 25, 2019 Page 1 of 11 May cause respiratory irritation. -

The Effect of High-Dose Thiotepa, Alone Or in Combination with Other
Bone Marrow Transplantation (2007) 40, 891–896 & 2007 Nature Publishing Group All rights reserved 0268-3369/07 $30.00 www.nature.com/bmt ORIGINAL ARTICLE The effect of high-dose thiotepa, alone or in combination with other chemotherapeutic agents, on a murine B-cell leukemia model simulating autologous stem cell transplantation A Abdul-Hai, L Weiss, D Ergas, IB Resnick, S Slavin and MY Shapira Department of Bone Marrow Transplantation and Cancer Immunotherapy, Hadassah–Hebrew University Medical Center, Jerusalem, Israel The use of thiotepa (TH) is increasing, especially in stem Introduction cell transplantation, mainly due to its safety and blood– brain barrier penetration. We evaluated the use of TH in a Thiotepa (TH, triethylene thiophosphoramide), an ethylene murine model simulating autologous stem cell transplan- amide, developed by Lederle Laboratories in 1952, tation, with or without additional agents. Between 1 and possesses mechlorethamine-like alkylating activity and 11 days following inoculation of BALB/c mice with 105– has been used clinically for over 35 years.1 It is of particular 108 B-cell leukemia (BCL1) cells (simulating pre-trans- use in breast cancer, mainly as second-line treatment.2 plant leukemia loads), each group received an ‘induction- TH has been given intrathecally for carcinomatous like’ irradiation and/or cytotoxic regimen. Animals were meningitis, and intravesically for bladder carcinoma.3,4 either followed without treatment, or an adoptive transfer Mild-to-moderate activity was observed in several solid (AT) was performed to untreated BALB/c mice. Admi- tumors and hematological malignancies.1,5 There has nistered alone without AT, high-dose TH did not change been, however, a sustained interest in defining clinical roles the time to appearance of leukemia. -

Thiirane-Terminated Polysulfide Polymers Thiiran-Terminierte Polysulfidpolymere Polymères Polysulfures À Terminaison Thiirane
(19) & (11) EP 2 121 807 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C08G 75/00 (2006.01) 26.05.2010 Bulletin 2010/21 (86) International application number: (21) Application number: 08707985.1 PCT/EP2008/050540 (22) Date of filing: 18.01.2008 (87) International publication number: WO 2008/090086 (31.07.2008 Gazette 2008/31) (54) THIIRANE-TERMINATED POLYSULFIDE POLYMERS THIIRAN-TERMINIERTE POLYSULFIDPOLYMERE POLYMÈRES POLYSULFURES À TERMINAISON THIIRANE (84) Designated Contracting States: • KOTTNER, Nils AT BE BG CH CY CZ DE DK EE ES FI FR GB GR D-78628 Rottweil (DE) HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT • ZEITLER, Michael RO SE SI SK TR D-53347 Alfter (DE) (30) Priority: 23.01.2007 EP 07101024 (74) Representative: Schalkwijk, Pieter Cornelis et al 02.02.2007 US 899273 P Akzo Nobel N.V. Intellectual Property Department (43) Date of publication of application: P.O. Box 9300 25.11.2009 Bulletin 2009/48 6800 SB Arnhem (NL) (73) Proprietor: Akzo Nobel N.V. (56) References cited: 6824 BM Arnhem (NL) WO-A-03/076487 WO-A1-2004/099283 JP-A- 2004 062 057 US-A- 5 173 549 (72) Inventors: • WITZEL, Silke D-42103 Wuppertal (DE) Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -

SUMMARY of PARTICULARLY HAZARDOUS SUBSTANCES (By
SUMMARY OF PARTICULARLY HAZARDOUS SUBSTANCES (by alpha) Key: SC -- Select Carcinogens RT -- Reproductive Toxins AT -- Acute Toxins SA -- Readily Absorbed Through the Skin DHS -- Chemicals of Interest Revised: 11/2012 ________________________________________________________ ___________ _ _ _ _ _ _ _ _ _ _ _ ||| | | | CHEMICAL NAME CAS # |SC|RT| AT | SA |DHS| ________________________________________________________ ___________ | _ | _ | _ | _ | __ | | | | | | | 2,4,5-T 000093-76-5 | | x | | x | | ABRIN 001393-62-0 | | | x | | | ACETALDEHYDE 000075-07-0 | x | | | | | ACETAMIDE 000060-35-5 | x | | | | | ACETOHYDROXAMIC ACID 000546-88-3 ||x| | x | | ACETONE CYANOHYDRIN, STABILIZED 000075-86-5 | | | x | | x | ACETYLAMINOFLUORENE,2- 000053-96-3 | x | | | | | ACID MIST, STRONG INORGANIC 000000-00-0 | x | | | | | ACROLEIN 000107-02-8 | | x | x | x | | ACRYLAMIDE 000079-06-1 | x | x | | x | | ACRYLONITRILE 000107-13-1 | x | x | x | x | | ACTINOMYCIN D 000050-76-0 ||x| | x | | ADIPONITRILE 000111-69-3 | | | x | | | ADRIAMYCIN 023214-92-8 | x | | | | | AFLATOXIN B1 001162-65-8 | x | | | | | AFLATOXIN M1 006795-23-9 | x | | | | | AFLATOXINS 001402-68-2 | x | | x | | | ALL-TRANS RETINOIC ACID 000302-79-4 | | x | | x | | ALPRAZOMAN 028981-97-7 | | x | | x | | ALUMINUM PHOSPHIDE 020859-73-8 | | | x | | x | AMANTADINE HYDROCHLORIDE 000665-66-7 | | x | | x | | AMINO-2,4-DIBROMOANTHRAQUINONE 000081-49-2 | x | | | | | AMINO-2-METHYLANTHRAQUINONE, 1- 000082-28-0 | x | | | | | AMINO-3,4-DIMETHYL-3h-IMIDAZO(4,5f)QUINOLINE,2- 077094-11-2 | x | | | | | AMINO-3,8-DIMETHYL-3H-IMIDAZO(4,5-f)QUINOXALINE, -
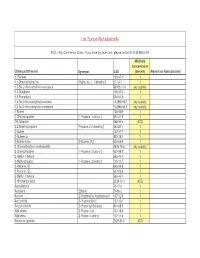
Homeland Security List
List Sorted Alphabetically ACG = Any Commercial Grade. If you have any questions, please contact EHS @ 898-5126. Minimum Concentration Chemical Of Interest Synonym CAS (percent) Amount on Hand (pounds) 1- Pentene 109-67-1 1 1,1-Dimethylhydrazine [Hydrazine, 1, 1-dimethyl-] 57-14-7 1 1,3-Bis(2-chloroethylthio)-n-propane 63905-10-2 any quantity 1,3-Butadiene 106-99-0 1 1,3-Pentadiene 504-60-9 1 1,4-Bis(2-chloroethylthio)-n-butane 142868-93-7 any quantity 1,5-Bis(2-chloroethylthio)-n-pentane 142868-94-8 any quantity 1-Butene 106-98-9 1 1-Chloropropylene [1-Propene, 1-chloro-] 590-21-6 1 1H-Tetrazole 288-94-8 ACG 2,2-Dimethylpropane [Propane, 2,2-dimethyl-] 463-82-1 1 2-Butene 107-01-7 1 2-Butene-cis 590-18-1 1 2-Butene-trans [2-Butene, (E)] 624-64-6 1 2-Chloroethylchloro-methylsulfide 2625-76-5 any quantity 2-Chloropropylene [1-Propene, 2-chloro-] 557-98-2 1 2-Methyl-1-butene 563-46-2 1 2-Methylpropene [1-Propene, 2-methyl-] 115-11-7 1 2-Pentene, (E)- 646-04-8 1 2-Pentene, (Z)- 627-20-3 1 3-Methyl-1-butene 563-45-1 1 5-Nitrobenzotriazol 2338-12-7 ACG Acetaldehyde 75-07-0 1 Acetylene [Ethyne] 74-86-2 1 Acrolein [2-Propenal] or Acrylaldehyde 107-02-8 1 Acrylonitrile [2-Propenenitrile] 107-13-1 1 Acrylyl chloride [2-Propenoyl chloride] 814-68-6 1 Allyl alcohol [2-Propen-1-ol] 107-18-6 1 Allylamine [2-Propen-1-amine] 107-11-9 1 Aluminum (powder) 7429-90-5 ACG Ammonia (anhydrous) 7664-41-7 1 Ammonia (conc. -
![Synthetic Approaches to Heterocyclic Bicyclo[2.1.0]Pentanes](https://docslib.b-cdn.net/cover/7157/synthetic-approaches-to-heterocyclic-bicyclo-2-1-0-pentanes-2177157.webp)
Synthetic Approaches to Heterocyclic Bicyclo[2.1.0]Pentanes
SYNTHETIC APPROACHES TO HETEROCYCLIC BICYCLO[2.1.0]PENTANES Rabah N. Alsulami A THESIS Submitted to the Graduate College of Bowling Green State University in partial fulfillment of The requirements for the degree of MASTER OF SCIENCE August 2011 Committee: Thomas H. Kinstle (Advisor) Marshall Wilson Alexander N. Tarnovsky ABSTRACT Thomas H. Kinstle, Advisor Bicyclic systems such as bicyclo[2.1.0]pentanes and 5-oxabicyclo[2.1.0]pentanes are known to display a variety of unique chemical properties associated with their high strain energy. To the best of our knowledge, there were no reports regarding synthesis and investigation of 5- azabicyclo[2.1.0]pentanes. Therefore, the initial goal of this research was synthesis of 5-azabicyclo[2.1.0]pentane and investigation of its chemical properties. The cycloaddition reaction of azides (58, 59, 61) to olefins (54, 55) with further elimination of nitrogen was chosen as a synthetic method in order to obtain the compounds of interest. Starting olefins (3,3-dimethyl-1-cyclobutene-1-carboxylic acid (54) and methyl 3,3-dimethyl-1-cyclobutene-1-carboxylate (55) and azides phenyl azide (58), p- toluenesulfonyl azide (59), and picryl azide (61) were successfully synthesized and characterized by NMR spectroscopy and GCMS spectrometry. The addition reaction between azides and olefins was performed under various conditions, such as different solvents and temperature; however, according to NMR spectroscopy and GCMS spectrometry, olefins (54, 55) do not undergo cycloaddition reaction with azides (58, 59, 61). In order to investigate that behavior, cycloaddition reactions of more reactive olefins (66, 68) with azides (58, 59, 61) were performed under a variety of conditions. -

Compendium Method TO-15
EPA/625/R-96/010b Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air Second Edition Compendium Method TO-15 Determination Of Volatile Organic Compounds (VOCs) In Air Collected In Specially-Prepared Canisters And Analyzed By Gas Chromatography/ Mass Spectrometry (GC/MS) Center for Environmental Research Information Office of Research and Development U.S. Environmental Protection Agency Cincinnati, OH 45268 January 1999 Method TO-15 Acknowledgements This Method was prepared for publication in the Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air, Second Edition (EPA/625/R-96/010b), which was prepared under Contract No. 68-C3-0315, WA No. 3-10, by Midwest Research Institute (MRI), as a subcontractor to Eastern Research Group, Inc. (ERG), and under the sponsorship of the U.S. Environmental Protection Agency (EPA). Justice A. Manning, John O. Burckle, and Scott Hedges, Center for Environmental Research Information (CERI), and Frank F. McElroy, National Exposure Research Laboratory (NERL), all in the EPA Office of Research and Development, were responsible for overseeing the preparation of this method. Additional support was provided by other members of the Compendia Workgroup, which include: • John O. Burckle, EPA, ORD, Cincinnati, OH • James L. Cheney, Corps of Engineers, Omaha, NB • Michael Davis, U.S. EPA, Region 7, KC, KS • Joseph B. Elkins Jr., U.S. EPA, OAQPS, RTP, NC • Robert G. Lewis, U.S. EPA, NERL, RTP, NC • Justice A. Manning, U.S. EPA, ORD, Cincinnati, OH • William A. McClenny, U.S. EPA, NERL, RTP, NC • Frank F. McElroy, U.S. EPA, NERL, RTP, NC • Heidi Schultz, ERG, Lexington, MA • William T. -

Aqua Regia Is a Mixture of Concentrated Nitric Acid and Hydrochloric Acid Most Commonly Used to Remove Trace Metals and TRACE Organic Materials from Glassware
Laboratory Safety Standard Operating Procedure (SOP) (For the use of hazardous materials or equipment) NAME OF PROCEDURE: AQUA REGIA PREPARED BY: RACHAEL MATTHEWS REVISION DATE: 07/16/2018 BRIEF DESCRIPTION OF PROCEDURE: 100 words or less, mass balanced equation, quantity limits Aqua Regia is a mixture of concentrated nitric acid and hydrochloric acid most commonly used to remove trace metals and TRACE organic materials from glassware. LOCATION – This procedure may be performed at the following location(s): Procedures are performed in fume hood only. HAZARDS – The materials and equipment associated with this procedure present the following exposure or physical health hazards. Safety precautions are prudent and mandatory (SDS of all chemicals referenced): -Aqua Regia is an oxidizer. Oxidizers are agents that initiate or promote combustion in other materials, generally through the release of oxygen. -Regia will oxidize over time to form toxic gases (nitrosyl chloride, nitrogen dioxide, and chlorine). The toxic gases formed as Aqua Regia oxidizes are also PHSs – nitrosyl chloride, nitrogen dioxide, and chlorine are all on the PHS list. Nitrosyl Chloride does not have a PEL or TLV. The PEL for nitrogen dioxide is 5 ppm (ceiling); the TLV is 3 ppm over 8 hours and 5 ppm for 15 minutes. The PEL for Chlorine gas is a 1 ppm (ceiling); the TLV is 0.5 ppm over 8 hours and 1 ppm for 15 minutes. -Aqua Regia solutions are extremely corrosive. Corrosive materials can cause destruction of living tissue by chemical action at the site of contact and can be solids, liquids, or gases. Corrosive effects can occur not only on the skin and eyes, but also in the respiratory tract and, in the case of ingestion, in the gastrointestinal tract as well. -

Anionic Polymerisation of Aziridines
MAX-PLANCK-INSTITUT FÜR POLYMERFORSCHUNG JOHANNES GUTENBERG‐UNIVERSITÄT MAINZ Anionic Polymerisation of Aziridines Diplomarbeit zur Erlangung des Grades eines Diplom‐Chemikers am Institut für Organische Chemie des Fachbereiches Chemie, Pharmazie und Geowissenschaften der Johannes Gutenberg-Universität Mainz vorgelegt von Laura Thomi geboren in Frankfurt am Main Mainz 2013 Diese Arbeit wurde in der Zeit von November 2012 bis Juli 2013 am Institut für Organische Chemie der Johannes Gutenberg‐Universität Mainz und am Max-Planck-Institut für Polymerforschung in Mainz unter der Betreuung von Herrn Prof. Dr. Holger Frey und Frau Prof. Dr. Katharina Landfester durchgeführt. für meine Eltern “We live on an island surrounded by a sea of ignorance. As our island of knowledge grows, so does the shore of our ignorance.” - John Archibald Wheeler Danksagung Mein Dank gilt Herrn Prof. Dr. Holger Frey und Frau Prof. Dr. Katharina Landfester für die Bereitstellung des Themas und die ausgezeichneten Arbeitsbedingungen. Zudem danke ich Herrn Dr. Frederik Wurm für die freundliche Aufnahme in seine Gruppe und die hervorragende Betreuung der Arbeit. Bei allen Mitgliedern der Arbeitsgruppe Frey bedanke ich mich für die wunderbare Arbeitsatmosphäre und die Unterstützung bei dieser Arbeit. Herrn Christian Moers und Herrn Jan Seiwert danke ich, dass sie mir die anionische Polymerisation von Styrol und Ethylenoxid näher gebracht haben. Bei Frau Anna Hesse und Herrn Christian Moers bedanke ich mich für das Korrekturlesen dieser Arbeit. Frau Katja Weber danke ich für die Bereitstellung des 2-Methyl-N-tosylaziridins. Für die GPC Messungen danke ich Frau Christine Rosenauer und insbesondere Frau Monika Schmelzer. Bei Frau Dr. Elena Berger-Nicoletti möchte ich mich für zahlreiche MALDI Messungen bedanken. -

TIH/PIH List
Hazardous Materials Designated as TIH/PIH (consolidated AAR and Railinc lists) 3/12/2007 STCC Proper Shipping Name 4921402 2-CHLOROETHANAL 4921495 2-METHYL-2-HEPTANETHIOL 4921741 3,5-DICHLORO-2,4,6-TRIFLUOROPYRIDINE 4921401 ACETONE CYANOHYDRIN, STABILIZED 4927007 ACROLEIN, STABILIZED 4921019 ALLYL ALCOHOL 4923113 ALLYL CHLOROFORMATE 4921004 ALLYLAMINE 4904211 AMMONIA SOLUTION 4920360 AMMONIA SOLUTIONS 4904209 AMMONIA, ANHYDROUS 4904210 AMMONIA, ANHYDROUS 4904879 AMMONIA, ANHYDROUS 4920359 AMMONIA, ANHYDROUS 4923209 ARSENIC TRICHLORIDE 4920135 ARSINE 4932010 BORON TRIBROMIDE 4920349 BORON TRICHLORIDE 4920522 BORON TRIFLUORIDE 4936110 BROMINE 4920715 BROMINE CHLORIDE 4918505 BROMINE PENTAFLUORIDE 4936106 BROMINE SOLUTIONS 4918507 BROMINE TRIFLUORIDE 4921727 BROMOACETONE 4920343 CARBON MONOXIDE AND HYDROGEN MIXTURE, COMPRESSED 4920399 CARBON MONOXIDE, COMPRESSED 4920511 CARBON MONOXIDE, REFRIGERATED LIQUID 4920559 CARBONYL FLUORIDE 4920351 CARBONYL SULFIDE 4920523 CHLORINE 4920189 CHLORINE PENTAFLUORIDE 4920352 CHLORINE TRIFLUORIDE 4921558 CHLOROACETONE, STABILIZED 4921009 CHLOROACETONITRILE 4923117 CHLOROACETYL CHLORIDE 4921414 CHLOROPICRIN 4920516 CHLOROPICRIN AND METHYL BROMIDE MIXTURES 4920547 CHLOROPICRIN AND METHYL BROMIDE MIXTURES 4920392 CHLOROPICRIN AND METHYL CHLORIDE MIXTURES 4921746 CHLOROPIVALOYL CHLORIDE 4930204 CHLOROSULFONIC ACID 4920527 COAL GAS, COMPRESSED 4920102 COMMPRESSED GAS, TOXIC, FLAMMABLE, CORROSIVE, N.O.S. 4920303 COMMPRESSED GAS, TOXIC, FLAMMABLE, CORROSIVE, N.O.S. 4920304 COMMPRESSED GAS, TOXIC, FLAMMABLE, CORROSIVE, N.O.S. 4920305 COMMPRESSED GAS, TOXIC, FLAMMABLE, CORROSIVE, N.O.S. 4920101 COMPRESSED GAS, TOXIC, CORROSIVE, N.O.S. 4920300 COMPRESSED GAS, TOXIC, CORROSIVE, N.O.S. 4920301 COMPRESSED GAS, TOXIC, CORROSIVE, N.O.S. 4920324 COMPRESSED GAS, TOXIC, CORROSIVE, N.O.S. 4920331 COMPRESSED GAS, TOXIC, CORROSIVE, N.O.S. 4920165 COMPRESSED GAS, TOXIC, FLAMMABLE, N.O.S. 4920378 COMPRESSED GAS, TOXIC, FLAMMABLE, N.O.S. 4920379 COMPRESSED GAS, TOXIC, FLAMMABLE, N.O.S.