Order GALLIFORMES
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Birds of Bharatpur – Check List
BIRDS OF BHARATPUR – CHECK LIST Family PHASIANIDAE: Pheasants, Partridges, Quail Check List BLACK FRANCOLIN GREY FRANCOLIN COMMON QUAIL RAIN QUAIL JUNGLE BUSH QUAIL YELLOW-LEGGED BUTTON QUAIL BARRED BUTTON QUAIL PAINTED SPURFOWL INDIAN PEAFOWL Family ANATIDAE: Ducks, Geese, Swans GREATER WHITE-FRONTED GOOSE GREYLAG GOOSE BAR-HEADED GOOSE LWSSER WHISTLING-DUCK RUDDY SHELDUCK COMMON SHELDUCK COMB DUCK COTTON PYGMY GOOSE MARBLED DUCK GADWALL FALCATED DUCK EURASIAN WIGEON MALLARD SPOT-BILLED DUCK COMMON TEAL GARGANEY NORTHERN PINTAIL NORTHERN SHOVELER RED-CRESTED POCHARD COMMON POCHARD FERRUGINOUS POCHARD TUFTED DUCK BAIKAL TEAL GREATER SCAUP BAER’S POCHARD Family PICIDAE: Woodpeckers EURASIAN WRYNECK BROWN-CAPPED PYGMY WOODPECKER YELLOW-CROWNED WOODPECKER BLACK-RUMPED FLAMBACK Family CAPITONIDAE: Barbets BROWN-HEADED BARBET COPPERSMITH BARBET Family UPUPIDAE: Hoopoes COMMON HOOPOE Family BUCEROTIDAE: Hornbills INDAIN GREY HORNBILL Family CORACIIDAE: Rollers or Blue Jays EUROPEAN ROLLER INDIAN ROLLER Family ALCEDINIDAE: Kingfisher COMMON KINGFISHER STORK-BILLED KINGFISHER WHITE-THROATED KINGFISHER BLACK-CAPPED KINGFISHER PIED KINGFISHER Family MEROPIDAE: Bee-eaters GREEN BEE-EATER BLUE-CHEEKED BEE-EATER BLUE-TAILED BEE-EATER Family CUCULIDAE: Cuckoos, Crow-pheasants PIED CUCKOO CHESTNUT-WINGED CUCKOO COMMON HAWK CUCKOO INDIAN CUCKOO EURASIAN CUCKOO GREY-BELLIED CUCKOO PLAINTIVE CUCKOO DRONGO CUCKOO ASIAN KOEL SIRKEER MALKOHA GREATER COUCAL LESSER COUCAL Family PSITTACIDAS: Parrots ROSE-RINGED PARAKEET PLUM-HEADED PARKEET Family APODIDAE: -

Introduction
Threatened Birds of Asia: The BirdLife International Red Data Book Editors N. J. COLLAR (Editor-in-chief), A. V. ANDREEV, S. CHAN, M. J. CROSBY, S. SUBRAMANYA and J. A. TOBIAS Maps by RUDYANTO and M. J. CROSBY Principal compilers and data contributors ■ BANGLADESH P. Thompson ■ BHUTAN R. Pradhan; C. Inskipp, T. Inskipp ■ CAMBODIA Sun Hean; C. M. Poole ■ CHINA ■ MAINLAND CHINA Zheng Guangmei; Ding Changqing, Gao Wei, Gao Yuren, Li Fulai, Liu Naifa, Ma Zhijun, the late Tan Yaokuang, Wang Qishan, Xu Weishu, Yang Lan, Yu Zhiwei, Zhang Zhengwang. ■ HONG KONG Hong Kong Bird Watching Society (BirdLife Affiliate); H. F. Cheung; F. N. Y. Lock, C. K. W. Ma, Y. T. Yu. ■ TAIWAN Wild Bird Federation of Taiwan (BirdLife Partner); L. Liu Severinghaus; Chang Chin-lung, Chiang Ming-liang, Fang Woei-horng, Ho Yi-hsian, Hwang Kwang-yin, Lin Wei-yuan, Lin Wen-horn, Lo Hung-ren, Sha Chian-chung, Yau Cheng-teh. ■ INDIA Bombay Natural History Society (BirdLife Partner Designate) and Sálim Ali Centre for Ornithology and Natural History; L. Vijayan and V. S. Vijayan; S. Balachandran, R. Bhargava, P. C. Bhattacharjee, S. Bhupathy, A. Chaudhury, P. Gole, S. A. Hussain, R. Kaul, U. Lachungpa, R. Naroji, S. Pandey, A. Pittie, V. Prakash, A. Rahmani, P. Saikia, R. Sankaran, P. Singh, R. Sugathan, Zafar-ul Islam ■ INDONESIA BirdLife International Indonesia Country Programme; Ria Saryanthi; D. Agista, S. van Balen, Y. Cahyadin, R. F. A. Grimmett, F. R. Lambert, M. Poulsen, Rudyanto, I. Setiawan, C. Trainor ■ JAPAN Wild Bird Society of Japan (BirdLife Partner); Y. Fujimaki; Y. Kanai, H. -

A Multi-Gene Phylogeny of Aquiline Eagles (Aves: Accipitriformes) Reveals Extensive Paraphyly at the Genus Level
Available online at www.sciencedirect.com MOLECULAR SCIENCE•NCE /W\/Q^DIRI DIRECT® PHYLOGENETICS AND EVOLUTION ELSEVIER Molecular Phylogenetics and Evolution 35 (2005) 147-164 www.elsevier.com/locate/ympev A multi-gene phylogeny of aquiline eagles (Aves: Accipitriformes) reveals extensive paraphyly at the genus level Andreas J. Helbig'^*, Annett Kocum'^, Ingrid Seibold^, Michael J. Braun^ '^ Institute of Zoology, University of Greifswald, Vogelwarte Hiddensee, D-18565 Kloster, Germany Department of Zoology, National Museum of Natural History, Smithsonian Institution, 4210 Silver Hill Rd., Suitland, MD 20746, USA Received 19 March 2004; revised 21 September 2004 Available online 24 December 2004 Abstract The phylogeny of the tribe Aquilini (eagles with fully feathered tarsi) was investigated using 4.2 kb of DNA sequence of one mito- chondrial (cyt b) and three nuclear loci (RAG-1 coding region, LDH intron 3, and adenylate-kinase intron 5). Phylogenetic signal was highly congruent and complementary between mtDNA and nuclear genes. In addition to single-nucleotide variation, shared deletions in nuclear introns supported one basal and two peripheral clades within the Aquilini. Monophyly of the Aquilini relative to other birds of prey was confirmed. However, all polytypic genera within the tribe, Spizaetus, Aquila, Hieraaetus, turned out to be non-monophyletic. Old World Spizaetus and Stephanoaetus together appear to be the sister group of the rest of the Aquilini. Spiza- stur melanoleucus and Oroaetus isidori axe nested among the New World Spizaetus species and should be merged with that genus. The Old World 'Spizaetus' species should be assigned to the genus Nisaetus (Hodgson, 1836). The sister species of the two spotted eagles (Aquila clanga and Aquila pomarina) is the African Long-crested Eagle (Lophaetus occipitalis). -

Wild Turkey Education Guide
Table of Contents Section 1: Eastern Wild Turkey Ecology 1. Eastern Wild Turkey Quick Facts………………………………………………...pg 2 2. Eastern Wild Turkey Fact Sheet………………………………………………….pg 4 3. Wild Turkey Lifecycle……………………………………………………………..pg 8 4. Eastern Wild Turkey Adaptations ………………………………………………pg 9 Section 2: Eastern Wild Turkey Management 1. Wild Turkey Management Timeline…………………….……………………….pg 18 2. History of Wild Turkey Management …………………...…..…………………..pg 19 3. Modern Wild Turkey Management in Maryland………...……………………..pg 22 4. Managing Wild Turkeys Today ……………………………………………….....pg 25 Section 3: Activity Lesson Plans 1. Activity: Growing Up WILD: Tasty Turkeys (Grades K-2)……………..….…..pg 33 2. Activity: Calling All Turkeys (Grades K-5)………………………………..…….pg 37 3. Activity: Fit for a Turkey (Grades 3-5)…………………………………………...pg 40 4. Activity: Project WILD adaptation: Too Many Turkeys (Grades K-5)…..…….pg 43 5. Activity: Project WILD: Quick, Frozen Critters (Grades 5-8).……………….…pg 47 6. Activity: Project WILD: Turkey Trouble (Grades 9-12………………….……....pg 51 7. Activity: Project WILD: Let’s Talk Turkey (Grades 9-12)..……………..………pg 58 Section 4: Additional Activities: 1. Wild Turkey Ecology Word Find………………………………………….…….pg 66 2. Wild Turkey Management Word Find………………………………………….pg 68 3. Turkey Coloring Sheet ..………………………………………………………….pg 70 4. Turkey Coloring Sheet ..………………………………………………………….pg 71 5. Turkey Color-by-Letter……………………………………..…………………….pg 72 6. Five Little Turkeys Song Sheet……. ………………………………………….…pg 73 7. Thankful Turkey…………………..…………………………………………….....pg 74 8. Graph-a-Turkey………………………………….…………………………….…..pg 75 9. Turkey Trouble Maze…………………………………………………………..….pg 76 10. What Animals Made These Tracks………………………………………….……pg 78 11. Drinking Straw Turkey Call Craft……………………………………….….……pg 80 Section 5: Wild Turkey PowerPoint Slide Notes The facilities and services of the Maryland Department of Natural Resources are available to all without regard to race, color, religion, sex, sexual orientation, age, national origin or physical or mental disability. -

A Baraminological Analysis of the Land Fowl (Class Aves, Order Galliformes)
Galliform Baraminology 1 Running Head: GALLIFORM BARAMINOLOGY A Baraminological Analysis of the Land Fowl (Class Aves, Order Galliformes) Michelle McConnachie A Senior Thesis submitted in partial fulfillment of the requirements for graduation in the Honors Program Liberty University Spring 2007 Galliform Baraminology 2 Acceptance of Senior Honors Thesis This Senior Honors Thesis is accepted in partial fulfillment of the requirements for graduation from the Honors Program of Liberty University. ______________________________ Timothy R. Brophy, Ph.D. Chairman of Thesis ______________________________ Marcus R. Ross, Ph.D. Committee Member ______________________________ Harvey D. Hartman, Th.D. Committee Member ______________________________ Judy R. Sandlin, Ph.D. Assistant Honors Program Director ______________________________ Date Galliform Baraminology 3 Acknowledgements I would like to thank my Lord and Savior, Jesus Christ, without Whom I would not have had the opportunity of being at this institution or producing this thesis. I would also like to thank my entire committee including Dr. Timothy Brophy, Dr. Marcus Ross, Dr. Harvey Hartman, and Dr. Judy Sandlin. I would especially like to thank Dr. Brophy who patiently guided me through the entire research and writing process and put in many hours working with me on this thesis. Finally, I would like to thank my family for their interest in this project and Robby Mullis for his constant encouragement. Galliform Baraminology 4 Abstract This study investigates the number of galliform bird holobaramins. Criteria used to determine the members of any given holobaramin included a biblical word analysis, statistical baraminology, and hybridization. The biblical search yielded limited biosystematic information; however, since it is a necessary and useful part of baraminology research it is both included and discussed. -

Partridges, Quails, Pheasants and Turkeys Phasianidae Vigors, 1825: Zoological Journal 2: 402 – Type Genus Phasianus Linnaeus, 1758
D .W . .5 / DY a 5D t w[ { wt Ç"" " !W5 í ÇI &'(' / b ù b a L w 5 ! ) " í "* " Ç t+ t " h " * { b ù" t* &)/&0 Order GALLIFORMES: Game Birds and Allies The order of galliform taxa in Checklist Committee (1990) appears to have been based on Peters (1934). Johnsgard (1986) synthesised available data, came up with similar groupings of taxa, and produced a dendrogram indicating that turkeys (Meleagridinae) were the most primitive (outside Cracidae and Megapodiidae), with grouse (Tetraoninae), guineafowl (Numidinae), New World quails (Odontophorinae) and pheasants and kin (Phasianinae) successively more derived. Genetic evidence (DNA-hybridisation data) provided by Sibley & Ahlquist (1990) suggested Odontophorinae were the most basal phasianoids and guineafowl the next most basal group. A basal position of the New World quails among phasianoids has been supported by other genetic data (Kimball et al. 1999, Armstrong et al. 2001). A recent analysis based on morphological characters (Dyke et al. 2003) found support for megapodes as the most basal group in the order, then Cracidae, then Phasianidoidea, and within the latter, Numididae the most basal group. In contrast to the above genetic-based analyses, Dyke et al. (2003) found the Odontophorinae to be the most derived group within the order. A recent analysis using both mitochondrial ND2 and cytochrome-b DNA sequences, however, reinforces the basal position of the Odontophorinae (Pereira & Baker 2006). Here we follow a consensus of the above works and place Odontophorinae basal in the phasianids. Worthy & Holdaway (2002) considered that Cheeseman’s (1891) second-hand record of megapodes from Raoul Island, Kermadec Group, before the 1870 volcanic eruption has veracity. -

A Molecular Phylogeny of the Peacock-Pheasants (Galliformes: Polyplectron Spp.) Indicates Loss and Reduction of Ornamental Traits and Display Behaviours
Biological Journal of the Linnean Society (2001), 73: 187–198. With 3 figures doi:10.1006/bijl.2001.0536, available online at http://www.idealibrary.com on A molecular phylogeny of the peacock-pheasants (Galliformes: Polyplectron spp.) indicates loss and reduction of ornamental traits and display behaviours REBECCA T. KIMBALL1,2∗, EDWARD L. BRAUN1,3, J. DAVID LIGON1, VITTORIO LUCCHINI4 and ETTORE RANDI4 1Department of Biology, University of New Mexico, Albuquerque, NM 87131, USA 2Department of Evolution, Ecology and Organismal Biology, Ohio State University, Columbus, OH 43210, USA 3Department of Plant Biology, Ohio State University, Columbus, OH 43210, USA 4Istituto Nazionale per la Fauna Selvatica, via Ca` Fornacetta 9, 40064 Ozzano dell’Emilia (BO), Italy Received 4 September 2000; accepted for publication 3 March 2001 The South-east Asian pheasant genus Polyplectron is comprised of six or seven species which are characterized by ocelli (ornamental eye-spots) in all but one species, though the sizes and distribution of ocelli vary among species. All Polyplectron species have lateral displays, but species with ocelli also display frontally to females, with feathers held erect and spread to clearly display the ocelli. The two least ornamented Polyplectron species, one of which completely lacks ocelli, have been considered the primitive members of the genus, implying that ocelli are derived. We examined this hypothesis phylogenetically using complete mitochondrial cytochrome b and control region sequences, as well as sequences from intron G in the nuclear ovomucoid gene, and found that the two least ornamented species are in fact the most recently evolved. Thus, the absence and reduction of ocelli and other ornamental traits in Polyplectronare recent losses. -
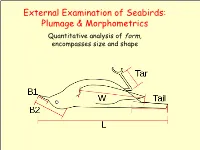
External Examination of Seabirds: Plumage & Morphometrics
External Examination of Seabirds: Plumage & Morphometrics Quantitative analysis of form, encompasses size and shape Seabird Topography ➢ Naming Conventions: • Parts of the body • Types of feathers (Harrison 1983) Types of Feathers Coverts: Rows bordering and overlaying the edges of the tail and wings on both the lower and upper sides of the body. Help streamline shape of the wings and tail and provide the bird with insulation. Feather Tracks ➢ Feathers are not attached randomly. • They occur in linear tracts called pterylae. • Spaces on bird's body without feather tracts are called apteria. • Densest area for feather tracks is head and neck. • Feathers arranged in distinct layers: contour feathers overlay down. Generic Pterylae Types of Feathers Contour feathers: outermost feathers. Define the color and shape of the bird. Contour feathers lie on top of each other, like shingles on a roof. Shed water, keeping body dry and insulated. Each contour feather controlled by specialized muscles which control their position, allowing the bird to keep the feathers in clean and neat condition. Specialized contour feathers used for flight: delineate outline of wings and tail. Types of Feathers Flight feathers – special contour feathers Define outline of wings and tail Long and stiff Asymmetrical those on wings are called remiges (singular remex) those on tail are called retrices (singular retrix) Types of Flight Feathers Remiges: Largest contour feathers (primaries / secondaries) Responsible for supporting bird during flight. Attached by ligaments or directly to the wing bone. Types of Flight Feathers Flight feathers – special contour feathers Rectrices: tail feathers provide flight stability and control. Connected to each other by ligaments, with only the inner- most feathers attached to bone. -

Hybridization & Zoogeographic Patterns in Pheasants
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Paul Johnsgard Collection Papers in the Biological Sciences 1983 Hybridization & Zoogeographic Patterns in Pheasants Paul A. Johnsgard University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/johnsgard Part of the Ornithology Commons Johnsgard, Paul A., "Hybridization & Zoogeographic Patterns in Pheasants" (1983). Paul Johnsgard Collection. 17. https://digitalcommons.unl.edu/johnsgard/17 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Paul Johnsgard Collection by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. HYBRIDIZATION & ZOOGEOGRAPHIC PATTERNS IN PHEASANTS PAUL A. JOHNSGARD The purpose of this paper is to infonn members of the W.P.A. of an unusual scientific use of the extent and significance of hybridization among pheasants (tribe Phasianini in the proposed classification of Johnsgard~ 1973). This has occasionally occurred naturally, as for example between such locally sympatric species pairs as the kalij (Lophura leucol11elana) and the silver pheasant (L. nycthelnera), but usually occurs "'accidentally" in captive birds, especially in the absence of conspecific mates. Rarely has it been specifically planned for scientific purposes, such as for obtaining genetic, morphological, or biochemical information on hybrid haemoglobins (Brush. 1967), trans ferins (Crozier, 1967), or immunoelectrophoretic comparisons of blood sera (Sato, Ishi and HiraI, 1967). The literature has been summarized by Gray (1958), Delacour (1977), and Rutgers and Norris (1970). Some of these alleged hybrids, especially those not involving other Galliformes, were inadequately doculnented, and in a few cases such as a supposed hybrid between domestic fowl (Gallus gal/us) and the lyrebird (Menura novaehollandiae) can be discounted. -

Bird-A-Thon San Diego County Team: Date
Stilts & Avocets Forster's Tern Red-tailed Hawk Bird-a-Thon Pheasants & Turkeys Black-necked Stilt Royal Tern Barn Owls Ring-necked Pheasant American Avocet Elegant Tern Barn Owl San Diego County Wild Turkey Plovers Black Skimmer Typical Owls Grebes Black-bellied Plover Loons Western Screech-Owl Pied-billed Grebe Snowy Plover Common Loon Great Horned Owl Team: Eared Grebe Semipalmated Plover Cormorants Burrowing Owl Western Grebe Killdeer Brandt's Cormorant Kingfishers Date: Clark's Grebe Sandpipers & Phalaropes Double-crested Cormorant Belted Kingfisher Ducks, Geese & Swans Pigeons & Doves Whimbrel Pelicans Rock Pigeon Brant Long-billed Curlew American White Pelican Woodpeckers Canada Goose Band-tailed Pigeon Marbled Godwit Brown Pelican Acorn Woodpecker Eurasian Collared-Dove Wood Duck Black Turnstone Bitterns, Herons & Egrets Downy Woodpecker Common Ground-Dove Blue-winged Teal Sanderling Great Blue Heron Nuttall's Woodpecker White-winged Dove Cinnamon Teal Least Sandpiper Great Egret Northern Flicker Mourning Dove Northern Shoveler Western Sandpiper Snowy Egret Caracaras & Falcons Cuckoos, Roadrunners & Anis Short-billed Dowitcher Little Blue Heron Gadwall American Kestrel Greater Roadrunner Eurasian Wigeon Long-billed Dowitcher Green Heron Peregrine Falcon Swifts American Wigeon Spotted Sandpiper Black-crowned Night-Heron New World Parrots Vaux's Swift Wandering Tattler Yellow-crowned Night-Heron Mallard Red-crowned Parrot White-throated Swift Northern Pintail Willet Ibises & Spoonbills Red-maked Parakeet Hummingbirds Green-winged -

Peafowl in Spring, Peacocks Begin Establishing Leks, Incubation Period Begins
may be impressed with the peacock’s beauty, Peahens conceal their nests, a simple scrape Visitor Centers & Recreation Services the true target of all his glamorous excess is lined with grass, in thick vegetation. The eggs ARDENWOOD HISTORIC FARM his potential mate, the peahen. are laid every other day until a total of up to Fremont 510-796-0199, [email protected] eight is reached, at which point the 28 day Peafowl In spring, peacocks begin establishing leks, incubation period begins. The peachicks are BLACK DIAMOND MINES a group of small adjacent territories, each of “precocial,” meaning they are born with their Antioch 925-757-2620, [email protected] which is the domain of a single male. Inter- eyes open, feathered, and ready to walk soon ested females visit the lek, assessing the vari- COYOTE HILLS REGIONAL PARK after hatching. Peahens are attentive mothers, Fremont 510-795-9385, [email protected] ous males before choosing a mate. In order but the peacock is not involved in raising his to attract mates, the peacock employs his young. The next generation of peahens will be CRAB COVE at CROWN BEACH most striking feature, the breathtaking train ready to reproduce at one or two years of age Alameda 510-521-6887, [email protected] of feathers often referred to as the peacock’s while the males need three years to develop tail. This 4 foot long collection of hundreds SUNOL REGIONAL WILDERNESS a full train of display plumage. Peafowl are Sunol 925-862-2601, [email protected] of feathers is actually made up of the upper hardy birds and may live 20 years or more. -

Bird) Species List
Aves (Bird) Species List Higher Classification1 Kingdom: Animalia, Phyllum: Chordata, Class: Reptilia, Diapsida, Archosauria, Aves Order (O:) and Family (F:) English Name2 Scientific Name3 O: Tinamiformes (Tinamous) F: Tinamidae (Tinamous) Great Tinamou Tinamus major Highland Tinamou Nothocercus bonapartei O: Galliformes (Turkeys, Pheasants & Quail) F: Cracidae Black Guan Chamaepetes unicolor (Chachalacas, Guans & Curassows) Gray-headed Chachalaca Ortalis cinereiceps F: Odontophoridae (New World Quail) Black-breasted Wood-quail Odontophorus leucolaemus Buffy-crowned Wood-Partridge Dendrortyx leucophrys Marbled Wood-Quail Odontophorus gujanensis Spotted Wood-Quail Odontophorus guttatus O: Suliformes (Cormorants) F: Fregatidae (Frigatebirds) Magnificent Frigatebird Fregata magnificens O: Pelecaniformes (Pelicans, Tropicbirds & Allies) F: Ardeidae (Herons, Egrets & Bitterns) Cattle Egret Bubulcus ibis O: Charadriiformes (Sandpipers & Allies) F: Scolopacidae (Sandpipers) Spotted Sandpiper Actitis macularius O: Gruiformes (Cranes & Allies) F: Rallidae (Rails) Gray-Cowled Wood-Rail Aramides cajaneus O: Accipitriformes (Diurnal Birds of Prey) F: Cathartidae (Vultures & Condors) Black Vulture Coragyps atratus Turkey Vulture Cathartes aura F: Pandionidae (Osprey) Osprey Pandion haliaetus F: Accipitridae (Hawks, Eagles & Kites) Barred Hawk Morphnarchus princeps Broad-winged Hawk Buteo platypterus Double-toothed Kite Harpagus bidentatus Gray-headed Kite Leptodon cayanensis Northern Harrier Circus cyaneus Ornate Hawk-Eagle Spizaetus ornatus Red-tailed