(AAD-1566) As a Potential Anticancer Agent in Ovarian Cancer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Analysis of Gene Expression Data for Gene Ontology
ANALYSIS OF GENE EXPRESSION DATA FOR GENE ONTOLOGY BASED PROTEIN FUNCTION PREDICTION A Thesis Presented to The Graduate Faculty of The University of Akron In Partial Fulfillment of the Requirements for the Degree Master of Science Robert Daniel Macholan May 2011 ANALYSIS OF GENE EXPRESSION DATA FOR GENE ONTOLOGY BASED PROTEIN FUNCTION PREDICTION Robert Daniel Macholan Thesis Approved: Accepted: _______________________________ _______________________________ Advisor Department Chair Dr. Zhong-Hui Duan Dr. Chien-Chung Chan _______________________________ _______________________________ Committee Member Dean of the College Dr. Chien-Chung Chan Dr. Chand K. Midha _______________________________ _______________________________ Committee Member Dean of the Graduate School Dr. Yingcai Xiao Dr. George R. Newkome _______________________________ Date ii ABSTRACT A tremendous increase in genomic data has encouraged biologists to turn to bioinformatics in order to assist in its interpretation and processing. One of the present challenges that need to be overcome in order to understand this data more completely is the development of a reliable method to accurately predict the function of a protein from its genomic information. This study focuses on developing an effective algorithm for protein function prediction. The algorithm is based on proteins that have similar expression patterns. The similarity of the expression data is determined using a novel measure, the slope matrix. The slope matrix introduces a normalized method for the comparison of expression levels throughout a proteome. The algorithm is tested using real microarray gene expression data. Their functions are characterized using gene ontology annotations. The results of the case study indicate the protein function prediction algorithm developed is comparable to the prediction algorithms that are based on the annotations of homologous proteins. -

Nucleolin and Its Role in Ribosomal Biogenesis
NUCLEOLIN: A NUCLEOLAR RNA-BINDING PROTEIN INVOLVED IN RIBOSOME BIOGENESIS Inaugural-Dissertation zur Erlangung des Doktorgrades der Mathematisch-Naturwissenschaftlichen Fakultät der Heinrich-Heine-Universität Düsseldorf vorgelegt von Julia Fremerey aus Hamburg Düsseldorf, April 2016 2 Gedruckt mit der Genehmigung der Mathematisch-Naturwissenschaftlichen Fakultät der Heinrich-Heine-Universität Düsseldorf Referent: Prof. Dr. A. Borkhardt Korreferent: Prof. Dr. H. Schwender Tag der mündlichen Prüfung: 20.07.2016 3 Die vorgelegte Arbeit wurde von Juli 2012 bis März 2016 in der Klinik für Kinder- Onkologie, -Hämatologie und Klinische Immunologie des Universitätsklinikums Düsseldorf unter Anleitung von Prof. Dr. A. Borkhardt und in Kooperation mit dem ‚Laboratory of RNA Molecular Biology‘ an der Rockefeller Universität unter Anleitung von Prof. Dr. T. Tuschl angefertigt. 4 Dedicated to my family TABLE OF CONTENTS 5 TABLE OF CONTENTS TABLE OF CONTENTS ............................................................................................... 5 LIST OF FIGURES ......................................................................................................10 LIST OF TABLES .......................................................................................................12 ABBREVIATION .........................................................................................................13 ABSTRACT ................................................................................................................19 ZUSAMMENFASSUNG -

A High-Throughput Approach to Uncover Novel Roles of APOBEC2, a Functional Orphan of the AID/APOBEC Family
Rockefeller University Digital Commons @ RU Student Theses and Dissertations 2018 A High-Throughput Approach to Uncover Novel Roles of APOBEC2, a Functional Orphan of the AID/APOBEC Family Linda Molla Follow this and additional works at: https://digitalcommons.rockefeller.edu/ student_theses_and_dissertations Part of the Life Sciences Commons A HIGH-THROUGHPUT APPROACH TO UNCOVER NOVEL ROLES OF APOBEC2, A FUNCTIONAL ORPHAN OF THE AID/APOBEC FAMILY A Thesis Presented to the Faculty of The Rockefeller University in Partial Fulfillment of the Requirements for the degree of Doctor of Philosophy by Linda Molla June 2018 © Copyright by Linda Molla 2018 A HIGH-THROUGHPUT APPROACH TO UNCOVER NOVEL ROLES OF APOBEC2, A FUNCTIONAL ORPHAN OF THE AID/APOBEC FAMILY Linda Molla, Ph.D. The Rockefeller University 2018 APOBEC2 is a member of the AID/APOBEC cytidine deaminase family of proteins. Unlike most of AID/APOBEC, however, APOBEC2’s function remains elusive. Previous research has implicated APOBEC2 in diverse organisms and cellular processes such as muscle biology (in Mus musculus), regeneration (in Danio rerio), and development (in Xenopus laevis). APOBEC2 has also been implicated in cancer. However the enzymatic activity, substrate or physiological target(s) of APOBEC2 are unknown. For this thesis, I have combined Next Generation Sequencing (NGS) techniques with state-of-the-art molecular biology to determine the physiological targets of APOBEC2. Using a cell culture muscle differentiation system, and RNA sequencing (RNA-Seq) by polyA capture, I demonstrated that unlike the AID/APOBEC family member APOBEC1, APOBEC2 is not an RNA editor. Using the same system combined with enhanced Reduced Representation Bisulfite Sequencing (eRRBS) analyses I showed that, unlike the AID/APOBEC family member AID, APOBEC2 does not act as a 5-methyl-C deaminase. -

Variation in Protein Coding Genes Identifies Information Flow
bioRxiv preprint doi: https://doi.org/10.1101/679456; this version posted June 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Animal complexity and information flow 1 1 2 3 4 5 Variation in protein coding genes identifies information flow as a contributor to 6 animal complexity 7 8 Jack Dean, Daniela Lopes Cardoso and Colin Sharpe* 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 Institute of Biological and Biomedical Sciences 25 School of Biological Science 26 University of Portsmouth, 27 Portsmouth, UK 28 PO16 7YH 29 30 * Author for correspondence 31 [email protected] 32 33 Orcid numbers: 34 DLC: 0000-0003-2683-1745 35 CS: 0000-0002-5022-0840 36 37 38 39 40 41 42 43 44 45 46 47 48 49 Abstract bioRxiv preprint doi: https://doi.org/10.1101/679456; this version posted June 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Animal complexity and information flow 2 1 Across the metazoans there is a trend towards greater organismal complexity. How 2 complexity is generated, however, is uncertain. Since C.elegans and humans have 3 approximately the same number of genes, the explanation will depend on how genes are 4 used, rather than their absolute number. -

Experimental Approaches in Delineating Mtor Signaling
G C A T T A C G G C A T genes Review Experimental Approaches in Delineating mTOR Signaling Jiayi Qian 1,2, Siyuan Su 1,2 and Pengda Liu 1,2,* 1 Lineberger Comprehensive Cancer Center, The University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; [email protected] (J.Q.); [email protected] (S.S.) 2 Department of Biochemistry and Biophysics, The University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA * Correspondence: [email protected]; Tel.: +01-919-966-3522 Received: 11 June 2020; Accepted: 30 June 2020; Published: 2 July 2020 Abstract: The mTOR signaling controls essential biological functions including proliferation, growth, metabolism, autophagy, ageing, and others. Hyperactivation of mTOR signaling leads to a plethora of human disorders; thus, mTOR is an attractive drug target. The discovery of mTOR signaling started from isolation of rapamycin in 1975 and cloning of TOR genes in 1993. In the past 27 years, numerous research groups have contributed significantly to advancing our understanding of mTOR signaling and mTOR biology. Notably, a variety of experimental approaches have been employed in these studies to identify key mTOR pathway members that shape up the mTOR signaling we know today. Technique development drives mTOR research, while canonical biochemical and yeast genetics lay the foundation for mTOR studies. Here in this review, we summarize major experimental approaches used in the past in delineating mTOR signaling, including biochemical immunoprecipitation approaches, genetic approaches, immunofluorescence microscopic approaches, hypothesis-driven studies, protein sequence or motif search driven approaches, and bioinformatic approaches. -

Agricultural University of Athens
ΓΕΩΠΟΝΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΑΘΗΝΩΝ ΣΧΟΛΗ ΕΠΙΣΤΗΜΩΝ ΤΩΝ ΖΩΩΝ ΤΜΗΜΑ ΕΠΙΣΤΗΜΗΣ ΖΩΙΚΗΣ ΠΑΡΑΓΩΓΗΣ ΕΡΓΑΣΤΗΡΙΟ ΓΕΝΙΚΗΣ ΚΑΙ ΕΙΔΙΚΗΣ ΖΩΟΤΕΧΝΙΑΣ ΔΙΔΑΚΤΟΡΙΚΗ ΔΙΑΤΡΙΒΗ Εντοπισμός γονιδιωματικών περιοχών και δικτύων γονιδίων που επηρεάζουν παραγωγικές και αναπαραγωγικές ιδιότητες σε πληθυσμούς κρεοπαραγωγικών ορνιθίων ΕΙΡΗΝΗ Κ. ΤΑΡΣΑΝΗ ΕΠΙΒΛΕΠΩΝ ΚΑΘΗΓΗΤΗΣ: ΑΝΤΩΝΙΟΣ ΚΟΜΙΝΑΚΗΣ ΑΘΗΝΑ 2020 ΔΙΔΑΚΤΟΡΙΚΗ ΔΙΑΤΡΙΒΗ Εντοπισμός γονιδιωματικών περιοχών και δικτύων γονιδίων που επηρεάζουν παραγωγικές και αναπαραγωγικές ιδιότητες σε πληθυσμούς κρεοπαραγωγικών ορνιθίων Genome-wide association analysis and gene network analysis for (re)production traits in commercial broilers ΕΙΡΗΝΗ Κ. ΤΑΡΣΑΝΗ ΕΠΙΒΛΕΠΩΝ ΚΑΘΗΓΗΤΗΣ: ΑΝΤΩΝΙΟΣ ΚΟΜΙΝΑΚΗΣ Τριμελής Επιτροπή: Aντώνιος Κομινάκης (Αν. Καθ. ΓΠΑ) Ανδρέας Κράνης (Eρευν. B, Παν. Εδιμβούργου) Αριάδνη Χάγερ (Επ. Καθ. ΓΠΑ) Επταμελής εξεταστική επιτροπή: Aντώνιος Κομινάκης (Αν. Καθ. ΓΠΑ) Ανδρέας Κράνης (Eρευν. B, Παν. Εδιμβούργου) Αριάδνη Χάγερ (Επ. Καθ. ΓΠΑ) Πηνελόπη Μπεμπέλη (Καθ. ΓΠΑ) Δημήτριος Βλαχάκης (Επ. Καθ. ΓΠΑ) Ευάγγελος Ζωίδης (Επ.Καθ. ΓΠΑ) Γεώργιος Θεοδώρου (Επ.Καθ. ΓΠΑ) 2 Εντοπισμός γονιδιωματικών περιοχών και δικτύων γονιδίων που επηρεάζουν παραγωγικές και αναπαραγωγικές ιδιότητες σε πληθυσμούς κρεοπαραγωγικών ορνιθίων Περίληψη Σκοπός της παρούσας διδακτορικής διατριβής ήταν ο εντοπισμός γενετικών δεικτών και υποψηφίων γονιδίων που εμπλέκονται στο γενετικό έλεγχο δύο τυπικών πολυγονιδιακών ιδιοτήτων σε κρεοπαραγωγικά ορνίθια. Μία ιδιότητα σχετίζεται με την ανάπτυξη (σωματικό βάρος στις 35 ημέρες, ΣΒ) και η άλλη με την αναπαραγωγική -

Insoluble Solids at High Concentrations Repress Yeast's Response
www.nature.com/scientificreports OPEN Insoluble solids at high concentrations repress yeast’s response against stress and Received: 4 June 2019 Accepted: 9 August 2019 increase intracellular ROS levels Published: xx xx xxxx Antonio D. Moreno 1, Cristina González-Fernández 2, Mercedes Ballesteros 1,2 & Elia Tomás-Pejó 2 Lignocellulosic ethanol production requires high substrate concentrations for its cost-competitiveness. This implies the presence of high concentrations of insoluble solids (IS) at the initial stages of the process, which may limit the fermentation performance of the corresponding microorganism. The presence of 40–60% IS (w/w) resulted in lower glucose consumption rates and reduced ethanol volumetric productivities of Saccharomyces cerevisiae F12. Yeast cells exposed to IS exhibited a wrinkled cell surface and a reduced mean cell size due to cavity formation. In addition, the intracellular levels of reactive oxygen species (ROS) increased up to 40%. These ROS levels increased up to 70% when both lignocellulose-derived inhibitors and IS were simultaneously present. The general stress response mechanisms (e.g. DDR2, TPS1 or ZWF1 genes, trehalose and glycogen biosynthesis, and DNA repair mechanisms) were found repressed, and ROS formation could not be counteracted by the induction of the genes involved in repairing the oxidative damage such as glutathione, thioredoxin and methionine scavenging systems (e.g. CTA1, GRX4, MXR1, and TSA1; and the repression of cell cycle progression, CLN3). Overall, these results clearly show the role of IS as an important microbial stress factor that afect yeast cells at physical, physiological, and molecular levels. Bioethanol fuel has increased in many countries as an efective alternative to reduce CO2 emissions in the trans- port sector. -
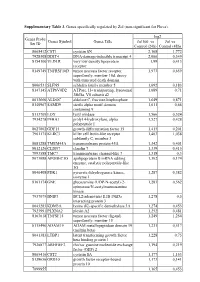
Supplementary Table 3. Genes Specifically Regulated by Zol (Non-Significant for Fluva)
Supplementary Table 3. Genes specifically regulated by Zol (non-significant for Fluva). log2 Genes Probe Genes Symbol Genes Title Zol100 vs Zol vs Set ID Control (24h) Control (48h) 8065412 CST1 cystatin SN 2,168 1,772 7928308 DDIT4 DNA-damage-inducible transcript 4 2,066 0,349 8154100 VLDLR very low density lipoprotein 1,99 0,413 receptor 8149749 TNFRSF10D tumor necrosis factor receptor 1,973 0,659 superfamily, member 10d, decoy with truncated death domain 8006531 SLFN5 schlafen family member 5 1,692 0,183 8147145 ATP6V0D2 ATPase, H+ transporting, lysosomal 1,689 0,71 38kDa, V0 subunit d2 8013660 ALDOC aldolase C, fructose-bisphosphate 1,649 0,871 8140967 SAMD9 sterile alpha motif domain 1,611 0,66 containing 9 8113709 LOX lysyl oxidase 1,566 0,524 7934278 P4HA1 prolyl 4-hydroxylase, alpha 1,527 0,428 polypeptide I 8027002 GDF15 growth differentiation factor 15 1,415 0,201 7961175 KLRC3 killer cell lectin-like receptor 1,403 1,038 subfamily C, member 3 8081288 TMEM45A transmembrane protein 45A 1,342 0,401 8012126 CLDN7 claudin 7 1,339 0,415 7993588 TMC7 transmembrane channel-like 7 1,318 0,3 8073088 APOBEC3G apolipoprotein B mRNA editing 1,302 0,174 enzyme, catalytic polypeptide-like 3G 8046408 PDK1 pyruvate dehydrogenase kinase, 1,287 0,382 isozyme 1 8161174 GNE glucosamine (UDP-N-acetyl)-2- 1,283 0,562 epimerase/N-acetylmannosamine kinase 7937079 BNIP3 BCL2/adenovirus E1B 19kDa 1,278 0,5 interacting protein 3 8043283 KDM3A lysine (K)-specific demethylase 3A 1,274 0,453 7923991 PLXNA2 plexin A2 1,252 0,481 8163618 TNFSF15 tumor necrosis -

Coexpression Networks Based on Natural Variation in Human Gene Expression at Baseline and Under Stress
University of Pennsylvania ScholarlyCommons Publicly Accessible Penn Dissertations Fall 2010 Coexpression Networks Based on Natural Variation in Human Gene Expression at Baseline and Under Stress Renuka Nayak University of Pennsylvania, [email protected] Follow this and additional works at: https://repository.upenn.edu/edissertations Part of the Computational Biology Commons, and the Genomics Commons Recommended Citation Nayak, Renuka, "Coexpression Networks Based on Natural Variation in Human Gene Expression at Baseline and Under Stress" (2010). Publicly Accessible Penn Dissertations. 1559. https://repository.upenn.edu/edissertations/1559 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/edissertations/1559 For more information, please contact [email protected]. Coexpression Networks Based on Natural Variation in Human Gene Expression at Baseline and Under Stress Abstract Genes interact in networks to orchestrate cellular processes. Here, we used coexpression networks based on natural variation in gene expression to study the functions and interactions of human genes. We asked how these networks change in response to stress. First, we studied human coexpression networks at baseline. We constructed networks by identifying correlations in expression levels of 8.9 million gene pairs in immortalized B cells from 295 individuals comprising three independent samples. The resulting networks allowed us to infer interactions between biological processes. We used the network to predict the functions of poorly-characterized human genes, and provided some experimental support. Examining genes implicated in disease, we found that IFIH1, a diabetes susceptibility gene, interacts with YES1, which affects glucose transport. Genes predisposing to the same diseases are clustered non-randomly in the network, suggesting that the network may be used to identify candidate genes that influence disease susceptibility. -
Gene Expression and Coexpression Alterations Marking Evolution of Bladder Cancer
medRxiv preprint doi: https://doi.org/10.1101/2021.06.15.21258890; this version posted June 21, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . 1 Rafael Stroggilos1,3, Maria Frantzi2, Jerome Zoidakis1, Emmanouil Mavrogeorgis1÷, Marika 2 Mokou2, Maria G Roubelakis3,4, Harald Mischak2, Antonia Vlahou1* 3 1. Systems Biology Center, Biomedical Research Foundation of the Academy of Athens, Athens, Greece. 4 2. Mosaiques Diagnostics GmbH, Hannover, Germany. 5 3. Laboratory of Biology, National and Kapodistrian University of Athens, School of Medicine, Athens, 6 Greece 7 4. Cell and Gene Therapy Laboratory, Biomedical Research Foundation of the Academy of Athens, Athens, 8 Greece. 9 ÷current address: Mosaiques Diagnostics GmbH, Hannover, Germany 10 * Corresponding author: 11 Antonia Vlahou, PhD 12 Biomedical Research Foundation, Academy of Athens 13 Soranou Efessiou 4, 14 11527, Athens, Greece 15 Tel: +30 210 6597506 16 Fax: +30 210 6597545 17 E-mail: [email protected] 18 19 TITLE 20 Gene expression and coexpression alterations marking evolution of bladder cancer 21 22 ABSTRACT 23 Despite advancements in therapeutics, Bladder Cancer (BLCA) constitutes a major clinical 24 burden, with locally advanced and metastatic cases facing poor survival rates. Aiming at 25 expanding our knowledge of BLCA molecular pathophysiology, we integrated 1,508 publicly 26 available, primary, well-characterized BLCA transcriptomes and investigated alterations in 27 gene expression with stage (T0-Ta-T1-T2-T3-T4). -

N6-Methyladenosine Mrna Marking Promotes Selective Translation of Regulons Required for Human Erythropoiesis
bioRxiv preprint doi: https://doi.org/10.1101/457648; this version posted October 31, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. N6-methyladenosine mRNA marking promotes selective translation of regulons required for human erythropoiesis Daniel A. Kuppers1, Sonali Arora1, Yiting Lim1, Andrea Lim1,2, Lucas Carter1, Philip Corrin1, Christopher L. Plaisier3, Ryan Basom4, Jeffrey J. Delrow4, Shiyan Wang5, Housheng Hansen He5, Beverly Torok-Storb6, Andrew C. Hsieh1,6,7* and Patrick J. Paddison1* Affiliations: 1Human Biology Division, Fred Hutchinson Cancer Research Center, Seattle, WA 98109, USA 2Molecular and Cellular Biology Program, University of Washington, Seattle, WA 98195, USA 3School of Biological and Health Systems Engineering, Arizona State University, Tempe, AZ 85281, USA 4Genomics Shared Resource, Fred Hutchinson Cancer Research Center, Seattle, WA 98109, USA 5Princess Margaret Cancer Centre/University Health Network, Toronto, Ontario, Canada 6Clinical Research Division, Fred Hutchinson Cancer Research Center, Seattle, WA 98109, USA 7School of Medicine, University of Washington, Seattle, WA 98195, USA *Correspondence to: [email protected] or [email protected] bioRxiv preprint doi: https://doi.org/10.1101/457648; this version posted October 31, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. Abstract Many of the regulatory features governing erythrocyte specification, maturation, and associated disorders remain enigmatic. -

Insights Into the Cellular Response Triggered by Silver Nanoparticles
Insights into the Cellular Response Triggered by Silver Nanoparticles using Quantitative Proteomics Thiago Verano-Braga, † Rona Miethling-Graff, ‡ Katarzyna Wojdyla, † Adelina Rogowska- Wrzesinska, † Jonathan R. Brewer, †† Helmut Erdmann, ‡ Frank Kjeldsen †* †Protein Research Group and †† MEMPHYS Center for Biomembrane Physics – Department of Biochemistry and Molecular Biology, University of Southern Denmark, Odense, Denmark ‡Biotechnology, University of Applied Science, Flensburg, Germany Supplementary Figure S1 – Proteomics reproducibility. In total, 3352 proteins were identified in this study using 3 independent biological replicates and 2524 proteins (75%) were identified in at least 2 replicates. Supplementary Figure S2 – Data normalization and significance analysis. (A) Boxplots for each of the 5 experimental conditions from the 3 independent biological replicates. Data was normalized by the median. (B) Histogram and (C) Q Q plot depicting the normal distribution of the dataset. (D) Volcano plot representing the p- values versus intensity changes for each experimental condition (20 nm AgNPs, 100 nm AgNPs, Ctrl 20 nm and Ctrl 100 nm). The p-values were corrected for multiple testing with Benjamini-Hochberg. Points above the dashed lines represent the proteins differentially regulated ( p < 0.01). Supplementary Figure S3 – Protein-protein interacting networks. Stronger interactions are represented by thicker lines. Over-represented biological functions based on gene ontology annotation are depicted in the figure. Confocal imaging – Confocal imaging was carried out on a Zeiss LSM 510 META. The samples were imaged using a 63 x 1.4 NA oil immersion objective (Carl Zeiss). For the 100 nm nanoparticles, the excitation laser was a 543 nm HeNe laser, they were imaged by detecting their reflection of the excitation laser.