Field Observations on the Mating Behavior of Aclees Sp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biology and Ecology of Leptographium Species and Their Vectos As Components of Loblolly Pine Decline Lori G
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2003 Biology and ecology of Leptographium species and their vectos as components of loblolly pine decline Lori G. Eckhardt Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Part of the Plant Sciences Commons Recommended Citation Eckhardt, Lori G., "Biology and ecology of Leptographium species and their vectos as components of loblolly pine decline" (2003). LSU Doctoral Dissertations. 2133. https://digitalcommons.lsu.edu/gradschool_dissertations/2133 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. BIOLOGY AND ECOLOGY OF LEPTOGRAPHIUM SPECIES AND THEIR VECTORS AS COMPONENTS OF LOBLOLLY PINE DECLINE A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Plant Pathology & Crop Physiology by Lori G. Eckhardt B.S., University of Maryland, 1997 August 2003 © Copyright 2003 Lori G. Eckhardt All rights reserved ii ACKNOWLEDGMENTS I gratefully acknowledge the invaluable input provided by my dissertation advisor, Dr. John P. Jones. Among many other things, he has demonstrated his patients, enthusiasm and understanding as I struggled to pursue my graduate studies. I am indebted to Dr. Marc A. Cohn, for his guidance, encouragement, support and most of all, his friendship. -

Title Ecological Studies on Dispersal Flight and Host Selection of The
Ecological studies on dispersal flight and host selection of the Title ambrosia beetle Platypus quercivorus (Murayama)( Dissertation_全文 ) Author(s) Pham, Duy Long Citation 京都大学 Issue Date 2020-09-23 URL https://doi.org/10.14989/doctor.k22787 許諾条件により本文は2021-04-01に公開; Pham DL, Ito Y, Okada R, Ikeno H, Isagi Y, Yamasaki M (2017) Phototactic behavior of the ambrosia beetle Platypus quercivorus (Murayama) (Coleoptera: Platypodidae) before and after flight. Journal of Insect Behavior 30(3): 318-330. doi: Right 10.1007/s10905-017-9615-3. Pham DL, Ito Y, Okada R, Ikeno H, Isagi Y, Yamasaki M (2019) Effects of leaf conditions and flight activity on the behaviour of Platypus quercivorus (Murayama) (Coleoptera: Platypodidae). Journal of Applied Entomology 143(9): 1000-1010. doi: 10.1111/jen.12671 Type Thesis or Dissertation Textversion ETD Kyoto University Ecological studies on dispersal flight and host selection of the ambrosia beetle Platypus quercivorus (Murayama) Pham Duy Long 2020 Table of contents Chapter 1: General introduction: Dispersal flight and host selection of bark and ambrosia beetles ........................................................................................................................... 1 Chapter 2: Factors affecting flight distance of Platypus quercivorus ............................ 19 Chapter 3: Effects of flight on phototactic behavior of Platypus quercivorus .............. 35 Chapter 4: Effects of leaf conditions on the olfactory response of Platypus quercivorus to leaf volatiles ........................................................................................................... -

R. L. Meagher, Jr.R and J. C. Kgaspi 2 Texas A&M University Agricultural
S O U T IIW E S T E R N E N T O M O L O G IS T N IA R .2003 M ttIIN ‐F IE L D D IST R IB U T IO N O F T H R E E H O M O PT E R A N SP E C ttS IN T E X A S SU G A R C A N E R. L. Meagher,Jr.r andJ. C. kgaspi 2 TexasA&M University Agricultural Researchand ExtensionCenter 2415EastHighway 83 Weslaco,Texas 78596 ABSTRACT Sugarcanefields composed ofeither'CP 70-321'or'NCo 310'weresampled during 1993 and 1994 for three speciesofhomopteran insects. The West Indian canefly, Saccharosydne saccharivora(tlestwood), wasthe mostabundant speiies collectedwith densitiesreaching over 40 per shoot. Populationdensities varied sigrificantly throughoutthe seasonin 1993but were similar in 1994. No differencein densitieswere found between sugarcane cultivars. Both the sugarcanedelphacid, Perhinsiella saccharicidaKirkaldyand the leafhopperDraeculacephala portola Ball, were found in low numbers(< 1.0per shoot). P. saccharicidareached its highest levelslater in the season,and 'CP 70-321'shoots harbored more individuals than 'NCo 310' shoots.Although D. portola dertsitieswere low, differe,ncesamong fields andbetwecn cultivars were found. Aggregation,as measuredusing Taylor a and b coefficients, was shown to be greaterfor ,S.saccharivora than the other species. INTRODUCTION Sugarcane(interspecific hybrids of Saccharum)has been commercially grown in a tlree- county areaof southemTexas since 1972. Stemboringpyralids, Mexican rice borer, Eoreuma loftini (Dyar), andsugarcane borer, Diatraea saccharalis(F.), havebeen the most seriousinsect pests of the in{ustry (Meagher et al. 1994); however, other insects are active in the Texas sugarcaneagroecos)Nstem. -

Conifer Bole Utilization by Wood-Boring Beetles in Western Oregon Cfatc75
CfAtc75-;,) ?-437`,, ) Conifer bole utilization by wood-boring beetles in western Oregon /p i H. ZHONG AND T. D. SCHOWALTER1 Department of Entomology, Oregon State University, Corvallis, OR 97331, U.S.A. Received December 7, 1988 Accepted April 4, 1989 ZHONG, H., and SCHOWALTER, T. D. 1989. Conifer bole utilization by wood-boring beetles in western Oregon. Can. J. For. Res. 19: 943-947. We studied wood excavation by scolytid and cerambycid beetles in decomposing boles of four conifer species during the first two years on the ground in western Oregon. Colonization density and gallery volumes were measured in experi- mental boles (0.5 m diameter x 5 m length) of Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco), western hemlock (Tsuga heterophylla (Raf.) Sarg.), Pacific silver fir (Abies amabilis (Dougl.) Forbes), and western red cedar ( Thuja plicata Donn). Ambrosia beetles (Coleoptera: Scolytidae) colonized boles only during the 1st year and were essentially restricted to Douglas-fir and western hemlock (removing 0.2% of the sapwood volume). Bark beetles (Coleoptera: Scolytidae) colonized boles only in the 1st year, primarily in Douglas-fir and Pacific silver fir (removing 7-8% of the phloem surface area). Wood borers (Coleoptera: Cerambycidae) excavated an additional 2.3% of the phloem surface area of Pacific silver fir in the 1st year and continued to excavate all species except Douglas-fir during the 2nd year. Consequences for the decomposition process are discussed. ZHONG, H., et SCHOWALTER, T. D. 1989. Conifer bole utilization by wood-boring beetles in western Oregon. Can. J. For. Res. 19 : 943-947. Lexcavation du bois par les coleopteres dans les troncs en decomposition de quatre especes de coniferes a ete etudiee durant les 2 premieres annees de leur position au sol dans louest de ]Oregon. -
Draft Clearwater Assessment: 5. Vegetative Resources
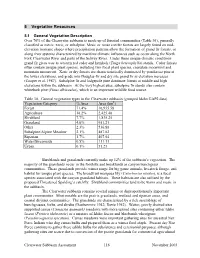
5 Vegetative Resources 5.1 General Vegetation Description Over 70% of the Clearwater subbasin is made up of forested communities (Table 30), generally classified as mesic, xeric, or subalpine. Mesic or moist conifer forests are largely found on mid- elevation montane slopes where precipitation patterns allow the formation of grand fir forests, or along river systems characterized by maritime climatic influences such as occur along the North Fork Clearwater River and parts of the Selway River. Under these unique climatic conditions grand fir gives way to western red cedar and hemlock (Tsuga heterophylla) stands. Cedar forests often contain unique plant species, including two focal plant species, crenulate moonwort and mountain moonwort. Xeric or dry forests are characteristically dominated by ponderosa pine at the lower elevations, and grade into Douglas-fir and dry site grand fir as elevation increases (Cooper et al. 1987). Subalpine fir and lodgepole pine dominate forests at middle and high elevations within the subbasin. At the very highest sites, subalpine fir stands also contain whitebark pine (Pinus albicaulus), which is an important wildlife food source. Table 30. General vegetation types in the Clearwater subbasin (grouped Idaho GAP2 data) Vegetation Category % Area Area (km2) Forest 71.4% 16,955.58 Agriculture 10.2% 2,425.48 Shrubland 7.7% 1,835.25 Grassland 4.0% 951.21 Other 2.3% 536.88 Subalpine/Alpine Meadow 2.1% 487.02 Riparian 1.7% 407.04 Water/Streamside 0.5% 111.11 Urban 0.1% 31.23 Shrublands and grasslands currently make up 12% of the subbasin’s vegetation. The majority of the grasslands occur in the foothills and breaklands as canyon bunchgrass communities. -

Effects of Pathogens and Bark Beetles on Forests
Utah State University DigitalCommons@USU Quinney Natural Resources Research Library, The Bark Beetles, Fuels, and Fire Bibliography S.J. and Jessie E. 1993 Effects of Pathogens and Bark Beetles on Forests D J. Goheen E M. Hansen Follow this and additional works at: https://digitalcommons.usu.edu/barkbeetles Part of the Ecology and Evolutionary Biology Commons, Entomology Commons, Forest Biology Commons, Forest Management Commons, and the Wood Science and Pulp, Paper Technology Commons Recommended Citation Goheen, D. and Hansen, E. Effects of pathogens and bark beetles on forests, pp. 175-196 in: TD Schowalter & GM Filip (eds) Beetle-Pathogen Interactions in Conifer Forests, Academic Press, London. This Contribution to Book is brought to you for free and open access by the Quinney Natural Resources Research Library, S.J. and Jessie E. at DigitalCommons@USU. It has been accepted for inclusion in The Bark Beetles, Fuels, and Fire Bibliography by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. .... 9.... Effects of Pathogens and Bark Beetles on Forests D. J. GOHEEN1 and E. M. HANSEN2 1USDA Forest Service, Pacific Northwest Region, Portland, OR, USA 2Department of Botany and Plant Pathology, Oregon State University, Corvallis, OR, USA 9.1 Introduction 175 9.2 Effects of Bark Beetle-Pathogen Interactions 176 9.2.1 Interactions in Pseudotsuga Forests: Phellinus and Deruiroctonus 176 9.2.2 Interactions in Pseudotsuga Forests: Leptographium and Bark Beetles 180 9.2.3 Interactions in Pinus ponderosa Forests 183 9.2.4 Interactions in Pinus contorta Forests 186 9.2.5 Interactions in Abies Forests 187 9.2.6 Interactions in Eastern Forests 190 9.3 Conclusions 191 References 191 9.1 INTRODUCTION Pathogenic fungi and bark beetles are important components of most coniferous forest ecosystems. -

A) Coleoptera: Curculionidae (Weevils) Family Characteristics: One of the Largest Animal Families with ~90,000 Described Species
A) Coleoptera: Curculionidae (weevils) Family characteristics: One of the largest animal families with ~90,000 described species. While there is considerable diversity in form and size, weevils have distinctive long snouts with chewing mouthparts at the end B and clubbed antennae. Steremnius carinatus A Conifer seedling weevil Economic importance: Adults feed at the base of vegetation (E). Clear cuts and site preparation encourage the weevil to feed on conifer seedlings. Economic injury has been worst on Vancouver Island and Haida Gwaii. Principal hosts: Seedlings of Douglas-fir, Sitka spruce C Hemlock and true firs are less preferred but sometimes attacked. 1mm Signs in the field: 1. Pupal cells at the base of seedlings (B, D) Notes : D E Adult Body Antennae Special Feature Injury Long and slender snout (C) Club shaped Flightless, adults Adult is injurious stage and obvious walk from host to Red-grey patterns on elytra Emerge from stumps and girdle host 7 – 10mm long seedlings (E) Larvae Body Injury Legless, curved (A) Larvae live in bark of stumps and slash from recent felling, where they feed on phloem White to pink, with brown head capsule Strong mandibles Images by: A), B) Terry Price, Georgia Forestry Commission, Bugwood.org C) USDA Forest Service - Northeastern Area Archive, USDA Forest Service, Bugwood.org D), E) http://www.forestry.ubc.ca/fetch21/FRST308/lab3/steremnius_carinatus/collar.html 1 Hylobius warreni A B Warren root collar weevil Economic importance: Feeding by larvae may cause girdling and subsequent tree mortality, as well as serious reductions in growth. May seriously hamper the establishment of pine D plantations. -

FIELD GUIDE to FOREST DAMAGE in British Columbia
FIELD GUIDE TO FOREST DAMAGE in British Columbia 3RD REVISED EDITION Field Guide to the Pests of Managed Forests in British Columbia (1983) Canadian Cataloguing in Publication Data The use of trade, firm, or corporation names in this publication is for the information and convenience of the reader. Such use does not constitute an official endorsement or approval by the Government of British Columbia of any product or service to the exclusion of any others that may also be suitable. Contents of this report are presented for discussion purposes only. Funding assistance does not imply endorsement of any statements or information contained herein by the Government of British Columbia. Uniform Resource Locators (urls), addresses, and contact information contained in this document are current at the time of printing unless otherwise noted. Print edition: ISBN 978-0-7726-6819-6 Electronic/PDF edition: ISBN 978-0-7726-6819-6 Citation Burleigh, J., T. Ebata, K.J. White, D. Rusch and H. Kope. (Eds.) 2014. Field Guide to Forest Damage in British Columbia (Joint publication, ISSN 0843-4719 ; no. 17) Authors’ affiliation Jennifer Burleigh, Tim Ebata and Harry Kope B.C. Ministry of Forests, Lands and Natural Resource Operations Resource Practices Branch, Victoria, B.C. Ken White B.C. Ministry of Forests, Lands and Natural Resource Operations Skeena Region, Smithers, B.C. David Rusch B.C. Ministry of Forests, Lands and Natural Resource Operations Cariboo Region, Williams Lake, B.C. Copies of this report may be obtained from: Crown Publications, Queen’s Printer PO Box 9452 Stn Prov Govt Victoria, BC v8w 9v7 1-800-663-6105 | www.crownpub.bc.ca For information on other publications in this series, visit www.for.gov.bc.ca/scripts/hfd/pubs/hfdcatalog/index.asp © 2014 Province of British Columbia When using information from this report, please cite fully and correctly. -

Western Redcedar
Thujaplicafa Donn ex D. Don Western Redcedar Cupressaceae Cypress family Don Minore Western redcedar (Thuja plicata), also called Pacific redcedar, giant-cedar, arborvitae, canoe-cedar, and shinglewood, is the only Thuja species native to western North America. Extant redcedar volumes are estimated to be 824 million m3 (29 billion R3) in British Columbia (43) and 228 million m3 (8 billion ft3) in the United States (16). Most of this volume is in mature trees, which have tapered, often-fluted bases, drooping branches, thin fibrous bark, and small scalelike leaves arrayed in flat sprays. Many have forked tops (fig. 1). They often reach ages of 800 to 1,000 years. One particularly large specimen in Washington has a d.b.h. of 592 cm (233 in), a height of 54.3 m (178 ft), and a crown spread of 16.5 m (54 ft). The wood is valuable and extensively used in a wide variety of products. Habitat Native Range Western redcedar grows along the Pacific coast from Humboldt County, CA (lat. 40” 10’ NJ, to the northern and western shores of Sumner Strait in southeastern Alaska (lat. 56” 30’ N.) (fig. 2). In California, it is common only in the lower Mad River drainage and the wet region south of Ferndale in Humboldt County; it is found elsewhere only in iso- lated stands in boggy habitats (19). North of the California-Oregon border, the coastal range broadens to include the western slopes of the Cas- cade Range north of Crater Lake and the eastern slopes north of about latitude 44” 30’ N. -

Forest Insect & Disease Management Guide for the Northern and Central
2009 Forest Health Protection and State Forestry Organizations Management Guide for Brennan A. Ferguson Ferguson Forest Black Stain Root Disease Pathology Consulting, Inc.; Missoula, MT Leptographium wageneri (Kendr.) Wingf. Hosts: This disease is common and damaging in Topics Douglas-fir and west of the Cascade range of Oregon, Ponderosa pine Washington and northeast California, and Introduction 1 Pinyon pine in parts of eastern Oregon. Nomenclature 2 Occasionally, other It rarely causes significant damage in Host infection 2 pine species. inland or Rocky Mountain forests. Pathological 2 anatomy Pathogenesis 3 Introduction Black stain root disease BSRD was detected in 18.6% of Symptoms 4 (BSRD) is a wilt-like disease of 500, 10- to 30-year-old Douglas-fir Pathogen survival 5 conifers caused by the native, plantations, compared to 1.2% of Insect vectors 5 insect-vectored, fungal pathogen same with Armillaria root disease Leptographium wageneri (Kendr.) and 7.0% with laminated root rot Soil disturbance 6 Wingf. (Harrington and Cobb (Hessburg et al. 2001). In a subset Precommercia 7 1983). It is considered one of the of eighty of the 500 plantations l thinning five most-damaging root diseases that underwent intensive BSRD in Western forests (Hadfield et al. surveys the percentage of crop Disease 8 management 1986). Black stain root disease is trees affected was: <0.1% in 93% widespread across much of the of stands; 0.1-2% in 3% of stands; References cited 9 range of its hosts, but incidence 2.1-5% in 3% of stands; and >5% and severity, and thus the in 1% of the stands (Hessburg et al. -

Hedera Helix) on the Arthropod Community of Stanley Park
Assessing the potential impact of English ivy (Hedera helix) on the arthropod community of Stanley Park by Tianbi Wu Honours Bachelor of Science, University of Toronto, 2017 Project Submitted in Partial Fulfilment of the Requirements for the Degree of Master of Science in the Ecological Restoration Program Faculty of Environment (SFU) and School of Construction and the Environment (BCIT) © Tianbi Wu 2020 SIMON FRASER UNIVERSITY BRITISH COLUMBIA INSTITUTE OF TECHNOLOGY 2020 Copyright in this work rests with the author. Please ensure that any reproduction or re-use is done in accordance with the relevant national copyright legislation. i Approval Name: Tianbi Wu Degree: Master of Science Title: Assessing the potential impact of English ivy (Hedera helix) on the arthropod community in Stanley Park Examining Committee: Supervisor and Chair Anayansi Cohen-Fernández Supervisor Faculty, BCIT Doug Ransome Examiner Faculty, BCIT Susan Owen Examiner Faculty, SFU Date Defended/Approved: April 15th, 2020 i Abstract English ivy (Hedera helix) is a vine species that had been introduced to North America in colonial times. Extensive monocultures of English ivy and the attachment to other plants have been shown to impact native flora of North America. Its impact on native fauna has been overlooked. I sampled arthropods in six native plant plots and six English ivy plots in Stanley Park, British Columbia. A weekly collection of arthropods through pitfall traps was conducted from May to August 2019. There was no significant difference in arthropod diversity and total abundance of groups between native and ivy plots. Non- metric multidimensional scaling was used to show distances between beetle community compositions. -

1 the Influence of Time of Precommercial Thinning on The
1 745 ;-^V The influence of time of precommercial thinning on the colonization of Douglas-fir by three species of root-colonizing insects J. J. WITCOSKY2 AND T. D. SCHOWALTER Department of Entomology, Oregon State University, Corvallis, OR, U.S.A. 97331 AND E. M. HANSEN Department of Botany and Plant Pathology, Oregon State University, Corvallis, OR, U.S.A. 97331 Received October 29, 19853 Accepted March 13, 1986 WITCOSKY, J. J., T. D. ScHowALTER, and E. M. HANSEN. 1986. The influence of time of precommercial thinning on the colonization of Douglas-fir by three species of root-colonizing insects. Can. J. For. Res. 16: 745-749. In plantations of Douglas-fir (Pseudorsuga menziesii (Mirb.) Franco) in western Oregon, precommercial thinning resulted in significantly increased abundances of insect vectors of black-stain root disease. Hylastes nigrinus (Mann.), Pissodes fasciatus LeC., and Steremnius carinatus (Boh.) were monitored for 2 years (1983 and 1984) in unthinned plots and in plots thinned in September 1982, January 1983, or May 1983. Abundances of these beetle species were significantly higher in thinned plots, relative to unthinned plots. However, the numbers of H. nigrinus and P. fasciatus caught by unbaited traps in plots thinned in May was reduced relative to plots thinned in September or January. These results su ggest that time of thinning can be manipulated to reduce the activity of these vectors in plantations that are mechanically thinned. WurcosKY, J. J., T. D. ScHowALTER et E. M. HANSEN. 1986. The influence of time of precommercial thinning on the colonization of Douglas-fir by three species of root-colonizing insects.