Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cladocera: Anomopoda: Daphniidae) from the Lower Cretaceous of Australia
Palaeontologia Electronica palaeo-electronica.org Ephippia belonging to Ceriodaphnia Dana, 1853 (Cladocera: Anomopoda: Daphniidae) from the Lower Cretaceous of Australia Thomas A. Hegna and Alexey A. Kotov ABSTRACT The first fossil ephippia (cladoceran exuvia containing resting eggs) belonging to the extant genus Ceriodaphnia (Anomopoda: Daphniidae) are reported from the Lower Cretaceous (Aptian) freshwater Koonwarra Fossil Bed (Strzelecki Group), South Gippsland, Victoria, Australia. They represent only the second record of (pre-Quater- nary) fossil cladoceran ephippia from Australia (Ceriodaphnia and Simocephalus, both being from Koonwarra). The occurrence of both of these genera is roughly coincident with the first occurrence of these genera elsewhere (i.e., Mongolia). This suggests that the early radiation of daphniid anomopods predates the breakup of Pangaea. In addi- tion, some putative cladoceran body fossils from the same locality are reviewed; though they are consistent with the size and shape of cladocerans, they possess no cladoceran-specific synapomorphies. They are thus regarded as indeterminate diplostracans. Thomas A. Hegna. Department of Geology, Western Illinois University, Macomb, IL 61455, USA. ta- [email protected] Alexey A. Kotov. A.N. Severtsov Institute of Ecology and Evolution, Leninsky Prospect 33, Moscow 119071, Russia and Kazan Federal University, Kremlevskaya Str.18, Kazan 420000, Russia. alexey-a- [email protected] Keywords: Crustacea; Branchiopoda; Cladocera; Anomopoda; Daphniidae; Cretaceous. Submission: 28 March 2016 Acceptance: 22 September 2016 INTRODUCTION tions that the sparse known fossil record does not correlate with a meager past diversity. The rarity of Water fleas (Crustacea: Cladocera) are small, the cladoceran fossils is probably an artifact, a soft-bodied branchiopod crustaceans and are a result of insufficient efforts to find them in known diverse and ubiquitous component of inland and new palaeontological collections (Kotov, aquatic communities (Dumont and Negrea, 2002). -

An Analysis of Primary and Secondary Production in Lake Kariba in a Changing Climate
AN ANALYSIS OF PRIMARY AND SECONDARY PRODUCTION IN LAKE KARIBA IN A CHANGING CLIMATE MZIME R. NDEBELE-MURISA A thesis submitted in partial fulfillment of the requirements for the degree of Doctor Philosophiae in the Department of Biodiversity and Conservation Biology, University of the Western Cape Supervisor: Prof. Charles Musil Co-Supervisor: Prof. Lincoln Raitt May 2011 An analysis of primary and secondary production in Lake Kariba in a changing climate Mzime Regina Ndebele-Murisa KEYWORDS Climate warming Limnology Primary production Phytoplankton Zooplankton Kapenta production Lake Kariba i Abstract Title: An analysis of primary and secondary production in Lake Kariba in a changing climate M.R. Ndebele-Murisa PhD, Biodiversity and Conservation Biology Department, University of the Western Cape Analysis of temperature, rainfall and evaporation records over a 44-year period spanning the years 1964 to 2008 indicates changes in the climate around Lake Kariba. Mean annual temperatures have increased by approximately 1.5oC, and pan evaporation rates by about 25%, with rainfall having declined by an average of 27.1 mm since 1964 at an average rate of 6.3 mm per decade. At the same time, lake water temperatures, evaporation rates, and water loss from the lake have increased, which have adversely affected lake water levels, nutrient and thermal dynamics. The most prominent influence of the changing climate on Lake Kariba has been a reduction in the lake water levels, averaging 9.5 m over the past two decades. These are associated with increased warming, reduced rainfall and diminished water and therefore nutrient inflow into the lake. The warmer climate has increased temperatures in the upper layers of lake water, the epilimnion, by an overall average of 1.9°C between 1965 and 2009. -

Appendix 3.6: Chronic Effects Benchmarks
October 2013 SHELL CANADA ENERGY Appendix 3.6: Chronic Effects Benchmarks Project Number: 13-1346-0001 REPORT APPENDIX 3.6: CHRONIC EFFECTS BENCHMARKS Table of Contents 1.0 INTRODUCTION ............................................................................................................................................................... 1 2.0 CHRONIC EFFECTS BENCHMARKS ............................................................................................................................. 1 2.1 Updated Canadian Council of Ministers of the Environment Protocol .................................................................. 2 2.2 Application ........................................................................................................................................................... 2 2.3 Screening of Constituents for Chronic Effects Benchmark Development ............................................................ 3 2.4 Assessment Methods .......................................................................................................................................... 6 2.4.1 General Approach .......................................................................................................................................... 6 2.5 Procedure ............................................................................................................................................................ 9 2.5.1 Step 1: Creation of a Toxicological Database ............................................................................................... -

Alternative Testing Approaches in Environmental Safety Assessment
Alternative Testing Approaches in Environmental Safety Assessment Technical Report No. 97 ISSN-0773-8072-97 Brussels, December 2005 Alternative Testing Approaches in Environmental Safety Assessment ECETOC TECHNICAL REPORT No. 97 © Copyright - ECETOC AISBL European Centre for Ecotoxicology and Toxicology of Chemicals 4 Avenue E. Van Nieuwenhuyse (Bte 6), B-1160 Brussels, Belgium. All rights reserved. No part of this publication may be reproduced, copied, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise without the prior written permission of the copyright holder. Applications to reproduce, store, copy or translate should be made to the Secretary General. ECETOC welcomes such applications. Reference to the document, its title and summary may be copied or abstracted in data retrieval systems without subsequent reference. The content of this document has been prepared and reviewed by experts on behalf of ECETOC with all possible care and from the available scientific information. It is provided for information only. ECETOC cannot accept any responsibility or liability and does not provide a warranty for any use or interpretation of the material contained in the publication. ECETOC TR No. 97 Alternative Testing Approaches in Environmental Safety Assessment Alternative Testing Approaches in Environmental Safety Assessment CONTENTS SUMMARY 1 1. INTRODUCTION 3 2. BACKGROUND 5 2.1 Definitions 5 2.1.1 Definition of a protected animal 5 2.1.2 Ethical considerations of animal use 6 2.1.3 The three Rs 6 2.2 Information needs 7 2.2.1 The use of fish in ecotoxicology 7 2.2.2 Numbers of fish used 8 2.2.3 Regulatory tests - chemicals 8 2.2.4 REACH and its impact on the use of fish 9 2.2.5 Regulatory tests - effluents 9 2.3 Potential for alternatives 10 2.3.1 Reduction 10 2.3.2 Refinement 11 2.3.3 Replacement 11 2.4 Report structure 13 2.4.1 Atmosphere 14 2.4.2 Terrestrial hazard assessment 14 3. -

Lightweight Django USING REST, WEBSOCKETS & BACKBONE
Lightweight Django USING REST, WEBSOCKETS & BACKBONE Julia Elman & Mark Lavin Lightweight Django LightweightDjango How can you take advantage of the Django framework to integrate complex “A great resource for client-side interactions and real-time features into your web applications? going beyond traditional Through a series of rapid application development projects, this hands-on book shows experienced Django developers how to include REST APIs, apps and learning how WebSockets, and client-side MVC frameworks such as Backbone.js into Django can power the new or existing projects. backend of single-page Learn how to make the most of Django’s decoupled design by choosing web applications.” the components you need to build the lightweight applications you want. —Aymeric Augustin Once you finish this book, you’ll know how to build single-page applications Django core developer, CTO, oscaro.com that respond to interactions in real time. If you’re familiar with Python and JavaScript, you’re good to go. “Such a good idea—I think this will lower the barrier ■ Learn a lightweight approach for starting a new Django project of entry for developers ■ Break reusable applications into smaller services that even more… the more communicate with one another I read, the more excited ■ Create a static, rapid prototyping site as a scaffold for websites and applications I am!” —Barbara Shaurette ■ Build a REST API with django-rest-framework Python Developer, Cox Media Group ■ Learn how to use Django with the Backbone.js MVC framework ■ Create a single-page web application on top of your REST API Lightweight ■ Integrate real-time features with WebSockets and the Tornado networking library ■ Use the book’s code-driven examples in your own projects Julia Elman, a frontend developer and tech education advocate, started learning Django in 2008 while working at World Online. -

Aquatic Toxicology MEES 743 3 Credits SPRING 2019 (Also Listed As TOX 625)
Aquatic Toxicology MEES 743 3 credits SPRING 2019 (also listed as TOX 625) Course Objectives / Overview This course will provide students with a broad perspective on the subject INSTRUCTOR DETAILS: of aquatic toxicology. It is a comprehensive course in which a definitive description of basic concepts and principles, laboratory testing and field Faculty Details: situations, as well as examples of typical data and their interpretation and Dr. Carys Mitchelmore use by industry and water resource managers, will be discussed. The fate [email protected] and toxicological action of environmental pollutants will be examined in 410-326-7283 aquatic ecosystems, whole organisms and at the cellular, biochemical and molecular levels. Current and emerging issues will be used as case studies CLASS MEETING DETAILS: throughout the course to illustrate specific ecosystems (e.g. Chesapeake Bay), pollution events (e.g. Deepwater Horizon Oil Spill), particular Date: Monday/Wednesday organisms (e.g. coral reefs) or a specific class of contaminants. Classes Time: 12-1.30pm will consist of lectures by the instructor together with some guest Originating Site: UMCES, CBL speakers in addition to group discussions. IVN bridge number: (800414) Phone call in number: (***) Expected Learning Outcomes Room phone number: CBL, Ed Houde Teaching suite Following completion of this course students will; (1) Have experience applying basic concepts in environmental COURSE TYPE: science, including environmental chemistry, biology and Check all that apply physiology, ecosystem health, management and regulatory issues, ☐ as they relate to pollution of aquatic ecosystems. Foundation (2) Be able to identify a current topic of concern and summarize ☐ Professional Development current data/papers in an oral presentation including directing an ☐ Issue Study Group open discussion with the rest of the class. -

Clam Dissection Guideline
Clam Dissection Guideline BACKGROUND: Clams are bivalves, meaning that they have shells consisting of two halves, or valves. The valves are joined at the top, and the adductor muscles on each side hold the shell closed. If the adductor muscles are relaxed, the shell is pulled open by ligaments located on each side of the umbo. The clam's foot is used to dig down into the sand, and a pair of long incurrent and excurrent siphons that extrude from the clam's mantle out the side of the shell reach up to the water above (only the exit points for the siphons are shown). Clams are filter feeders. Water and food particles are drawn in through one siphon to the gills where tiny, hair-like cilia move the water, and the food is caught in mucus on the gills. From there, the food-mucus mixture is transported along a groove to the palps (mouth flaps) which push it into the clam's mouth. The second siphon carries away the water. The gills also draw oxygen from the water flow. The mantle, a thin membrane surrounding the body of the clam, secretes the shell. The oldest part of the clam shell is the umbo, and it is from the hinge area that the clam extends as it grows. I. Purpose: The purpose of this lab is to identify the internal and external structures of a mollusk by dissecting a clam. II. Materials: 2 pairs of safety goggles 1 paper towel 2 pairs of gloves 1 pair of scissors 1 preserved clam 2 pairs of forceps 1 dissecting tray 2 probes III. -

AN INTRODUCTION to AQUATIC TOXICOLOGY This Page Intentionally Left Blank ÂÂ an INTRODUCTION to AQUATIC TOXICOLOGY
AN INTRODUCTION TO AQUATIC TOXICOLOGY This page intentionally left blank AN INTRODUCTION TO AQUATIC TOXICOLOGY MIKKO NIKINMAA Professor of Zoology, Department of Biology, Laboratory of Animal Physiology, University of Turku, Turku, Finland AMSTERDAM • BOSTON • HEIDELBERG • LONDON NEW YORK • OXFORD • PARIS • SAN DIEGO SAN FRANCISCO • SINGAPORE • SYDNEY • TOKYO Academic press is an imprint of Elsevier Academic Press is an imprint of Elsevier The Boulevard, Langford Lane, Kidlington, Oxford, OX5 1GB, UK 225 Wyman Street, Waltham, MA 02451, USA Copyright © 2014 Elsevier Inc. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. Details on how to seek permission, further information about the Publisher’s permissions policies and our arrangement with organizations such as the Copyright Clearance Center and the Copyright Licensing Agency, can be found at our website: www.elsevier.com/permissions This book and the individual contributions contained in it are protected under copyright by the Publisher (other than as may be noted herein). Notices Knowledge and best practice in this field are constantly changing. As new research and experience broaden our understanding, changes in research methods, professional practices, or medical treatment may become necessary. Practitioners and researchers must always rely on their own experience and knowledge in evaluating and using any information, methods, compounds, or experiments described herein. In using such information or methods they should be mindful of their own safety and the safety of others, including parties for whom they have a professional responsibility. -

Harmful Algal Blooms (Habs) and Public Health: Progress and Current Challenges
Harmful Algal Blooms (HABs) and Public Health: Progress and Current Challenges Edited by Lesley V. D’Anglada, Elizabeth D. Hilborn and Lorraine C. Backer Printed Edition of the Special Issue Published in Toxins www.mdpi.com/journal/toxins Lesley V. D’Anglada, Elizabeth D. Hilborn and Lorraine C. Backer (Eds.) Harmful Algal Blooms (HABs) and Public Health: Progress and Current Challenges This book is a reprint of the Special Issue that appeared in the online, open access journal, Toxins (ISSN 2072-6651) from 2014–2015 (available at: http://www.mdpi.com/journal/toxins/special_issues/HABs?sort=asc). Guest Editors Lesley V. D’Anglada U.S. Environmental Protection Agency USA Elizabeth D. Hilborn United States Environmental Protection Agency USA Lorraine C. Backer National Center for Environmental Health USA Editorial Office MDPI AG Klybeckstrasse 64 Basel, Switzerland Publisher Shu-Kun Lin Managing Editor Chao Xiao 1. Edition 2016 MDPI • Basel • Beijing • Wuhan • Barcelona ISBN 978-3-03842-155-9 (Hbk) ISBN 978-3-03842-156-6 (PDF) © 2016 by the authors; licensee MDPI, Basel, Switzerland. All articles in this volume are Open Access distributed under the Creative Commons Attribution license (CC BY), which allows users to download, copy and build upon published articles even for commercial purposes, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications. However, the dissemination and distribution of physical copies of this book as a whole is restricted to MDPI, Basel, Switzerland. III Table of Contents List of Contributors ............................................................................................................ VII About the Guest Editors......................................................................................................... X Preface Reprinted from: Toxins 2015, 7, 4437-4441 http://www.mdpi.com/2072-6651/7/11/4437 .................................................................... -

The Benthic Feeding Ecology of Round Goby Fry Dylan Samuel Olson University of Wisconsin-Milwaukee
University of Wisconsin Milwaukee UWM Digital Commons Theses and Dissertations August 2016 The Benthic Feeding Ecology of Round Goby Fry Dylan Samuel Olson University of Wisconsin-Milwaukee Follow this and additional works at: https://dc.uwm.edu/etd Part of the Ecology and Evolutionary Biology Commons Recommended Citation Olson, Dylan Samuel, "The Benthic Feeding Ecology of Round Goby Fry" (2016). Theses and Dissertations. 1397. https://dc.uwm.edu/etd/1397 This Thesis is brought to you for free and open access by UWM Digital Commons. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of UWM Digital Commons. For more information, please contact [email protected]. THE BENTHIC FEEDING ECOLOGY OF ROUND GOBY FRY by Dylan S. Olson A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science in Freshwater Sciences and Technology at The University of Wisconsin-Milwaukee August 2016 ABSTRACT THE BENTHIC FEEDING ECOLOGY OF ROUND GOBY FRY by Dylan S. Olson The University of Wisconsin-Milwaukee, 2016 Under the Supervision of Professor John Janssen Larval and juvenile stage events play a dominant role in regulating the ultimate recruitment strength of fish populations. As such, the feeding ecology of early life stages are useful for interpreting the proximate causes of recruitment variability. This study provides the first targeted study of the early juvenile (“fry”) diet of the round goby (Neogobius melanostomus, Pallas 1814), a prominent Great Lakes invasive fish. Previous accounts of the diets of round goby fry in the Great Lakes have been based upon by-catch from nocturnal, pelagic studies. -
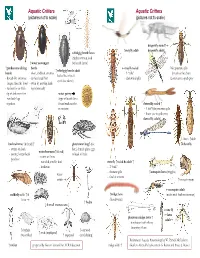
Aquatic Critters Aquatic Critters (Pictures Not to Scale) (Pictures Not to Scale)
Aquatic Critters Aquatic Critters (pictures not to scale) (pictures not to scale) dragonfly naiad↑ ↑ mayfly adult dragonfly adult↓ whirligig beetle larva (fairly common look ↑ water scavenger for beetle larvae) ↑ predaceous diving beetle mayfly naiad No apparent gills ↑ whirligig beetle adult beetle - short, clubbed antenna - 3 “tails” (breathes thru butt) - looks like it has 4 - thread-like antennae - surface head first - abdominal gills Lower jaw to grab prey eyes! (see above) longer than the head - swim by moving hind - surface for air with legs alternately tip of abdomen first water penny -row bklback legs (fbll(type of beetle larva together found under rocks damselfly naiad ↑ in streams - 3 leaf’-like posterior gills - lower jaw to grab prey damselfly adult↓ ←larva ↑adult backswimmer (& head) ↑ giant water bug↑ (toe dobsonfly - swims on back biter) female glues eggs water boatman↑(&head) - pointy, longer beak to back of male - swims on front -predator - rounded, smaller beak stonefly ↑naiad & adult ↑ -herbivore - 2 “tails” - thoracic gills ↑mosquito larva (wiggler) water - find in streams strider ↑mosquito pupa mosquito adult caddisfly adult ↑ & ↑midge larva (males with feather antennae) larva (bloodworm) ↑ hydra ↓ 4 small crustaceans ↓ crane fly ←larva phantom midge larva ↑ adult→ - translucent with silvery bflbuoyancy floats ↑ daphnia ↑ ostracod ↑ scud (amphipod) (water flea) ↑ copepod (seed shrimp) References: Aquatic Entomology by W. Patrick McCafferty ↑ rotifer prepared by Gwen Heistand for ACR Education midge adult ↑ Guide to Microlife by Kenneth G. Rainis and Bruce J. Russel 28 How do Aquatic Critters Get Their Air? Creeks are a lotic (flowing) systems as opposed to lentic (standing, i.e, pond) system. Look for … BREATHING IN AN AQUATIC ENVIRONMENT 1. -

Iep Newsletter
n Interagency Ecological Program for the San Francisco Estuary n IEP NEWSLETTER VOLUME 30, NUMBER 1, 2017 OF INTEREST TO MANAGERS .......................................................................................................................... 2 HIGHLIGHTS .......................................................................................................................................................... 3 All Hands on Deck: Revamping the Discrete Water Quality Blueprints of the IEP’s Environmental Monitoring Program .......................................................................................................................... 3 Summary of DWR Old-Middle River Turbidity Transects ........................................................................................ 5 STATUS AND TRENDS .......................................................................................................................................... 9 Zooplankton Monitoring 2013–2015 ......................................................................................................................... 9 CONTRIBUTED PAPERS .................................................................................................................................... 21 Evaluation of Adding Index Stations in Calculating the 20-mm Survey Delta Smelt Abundance Index ................ 21 Evaluating Potential Impact of Fish Removal at the Salvage Facility as part of the Delta Smelt Resiliency Strategy ..............................................................................................................................