Lewis Structures and the Localized Electron Bonding Model
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Five Common Particles
The Five Common Particles The world around you consists of only three particles: protons, neutrons, and electrons. Protons and neutrons form the nuclei of atoms, and electrons glue everything together and create chemicals and materials. Along with the photon and the neutrino, these particles are essentially the only ones that exist in our solar system, because all the other subatomic particles have half-lives of typically 10-9 second or less, and vanish almost the instant they are created by nuclear reactions in the Sun, etc. Particles interact via the four fundamental forces of nature. Some basic properties of these forces are summarized below. (Other aspects of the fundamental forces are also discussed in the Summary of Particle Physics document on this web site.) Force Range Common Particles It Affects Conserved Quantity gravity infinite neutron, proton, electron, neutrino, photon mass-energy electromagnetic infinite proton, electron, photon charge -14 strong nuclear force ≈ 10 m neutron, proton baryon number -15 weak nuclear force ≈ 10 m neutron, proton, electron, neutrino lepton number Every particle in nature has specific values of all four of the conserved quantities associated with each force. The values for the five common particles are: Particle Rest Mass1 Charge2 Baryon # Lepton # proton 938.3 MeV/c2 +1 e +1 0 neutron 939.6 MeV/c2 0 +1 0 electron 0.511 MeV/c2 -1 e 0 +1 neutrino ≈ 1 eV/c2 0 0 +1 photon 0 eV/c2 0 0 0 1) MeV = mega-electron-volt = 106 eV. It is customary in particle physics to measure the mass of a particle in terms of how much energy it would represent if it were converted via E = mc2. -

Brief Guide to the Nomenclature of Organic Chemistry
1 Brief Guide to the Nomenclature of Table 1: Components of the substitutive name Organic Chemistry (4S,5E)-4,6-dichlorohept-5-en-2-one for K.-H. Hellwich (Germany), R. M. Hartshorn (New Zealand), CH3 Cl O A. Yerin (Russia), T. Damhus (Denmark), A. T. Hutton (South 4 2 Africa). E-mail: [email protected] Sponsoring body: Cl 6 CH 5 3 IUPAC Division of Chemical Nomenclature and Structure suffix for principal hept(a) parent (heptane) one Representation. characteristic group en(e) unsaturation ending chloro substituent prefix 1 INTRODUCTION di multiplicative prefix S E stereodescriptors CHEMISTRY The universal adoption of an agreed nomenclature is a key tool for 2 4 5 6 locants ( ) enclosing marks efficient communication in the chemical sciences, in industry and Multiplicative prefixes (Table 2) are used when more than one for regulations associated with import/export or health and safety. fragment of a particular kind is present in a structure. Which kind of REPRESENTATION The International Union of Pure and Applied Chemistry (IUPAC) multiplicative prefix is used depends on the complexity of the provides recommendations on many aspects of nomenclature.1 The APPLIED corresponding fragment – e.g. trichloro, but tris(chloromethyl). basics of organic nomenclature are summarized here, and there are companion documents on the nomenclature of inorganic2 and Table 2: Multiplicative prefixes for simple/complicated entities polymer3 chemistry, with hyperlinks to original documents. An No. Simple Complicated No. Simple Complicated AND overall -

A Discussion on Characteristics of the Quantum Vacuum
A Discussion on Characteristics of the Quantum Vacuum Harold \Sonny" White∗ NASA/Johnson Space Center, 2101 NASA Pkwy M/C EP411, Houston, TX (Dated: September 17, 2015) This paper will begin by considering the quantum vacuum at the cosmological scale to show that the gravitational coupling constant may be viewed as an emergent phenomenon, or rather a long wavelength consequence of the quantum vacuum. This cosmological viewpoint will be reconsidered on a microscopic scale in the presence of concentrations of \ordinary" matter to determine the impact on the energy state of the quantum vacuum. The derived relationship will be used to predict a radius of the hydrogen atom which will be compared to the Bohr radius for validation. The ramifications of this equation will be explored in the context of the predicted electron mass, the electrostatic force, and the energy density of the electric field around the hydrogen nucleus. It will finally be shown that this perturbed energy state of the quan- tum vacuum can be successfully modeled as a virtual electron-positron plasma, or the Dirac vacuum. PACS numbers: 95.30.Sf, 04.60.Bc, 95.30.Qd, 95.30.Cq, 95.36.+x I. BACKGROUND ON STANDARD MODEL OF COSMOLOGY Prior to developing the central theme of the paper, it will be useful to present the reader with an executive summary of the characteristics and mathematical relationships central to what is now commonly referred to as the standard model of Big Bang cosmology, the Friedmann-Lema^ıtre-Robertson-Walker metric. The Friedmann equations are analytic solutions of the Einstein field equations using the FLRW metric, and Equation(s) (1) show some commonly used forms that include the cosmological constant[1], Λ. -

Valence Bond Theory
UMass Boston, Chem 103, Spring 2006 CHEM 103 Molecular Geometry and Valence Bond Theory Lecture Notes May 2, 2006 Prof. Sevian Announcements z The final exam is scheduled for Monday, May 15, 8:00- 11:00am It will NOT be in our regularly scheduled lecture hall (S- 1-006). The final exam location has been changed to Snowden Auditorium (W-1-088). © 2006 H. Sevian 1 UMass Boston, Chem 103, Spring 2006 More announcements Information you need for registering for the second semester of general chemistry z If you will take it in the summer: z Look for chem 104 in the summer schedule (includes lecture and lab) z If you will take it in the fall: z Look for chem 116 (lecture) and chem 118 (lab). These courses are co-requisites. z If you plan to re-take chem 103, in the summer it will be listed as chem 103 (lecture + lab). In the fall it will be listed as chem 115 (lecture) + chem 117 (lab), which are co-requisites. z Note: you are only eligible for a lab exemption if you previously passed the course. Agenda z Results of Exam 3 z Molecular geometries observed z How Lewis structure theory predicts them z Valence shell electron pair repulsion (VSEPR) theory z Valence bond theory z Bonds are formed by overlap of atomic orbitals z Before atoms bond, their atomic orbitals can hybridize to prepare for bonding z Molecular geometry arises from hybridization of atomic orbitals z σ and π bonding orbitals © 2006 H. Sevian 2 UMass Boston, Chem 103, Spring 2006 Molecular Geometries Observed Tetrahedral See-saw Square planar Square pyramid Lewis Structure Theory -

Lesson 1: the Single Electron Atom: Hydrogen
Lesson 1: The Single Electron Atom: Hydrogen Irene K. Metz, Joseph W. Bennett, and Sara E. Mason (Dated: July 24, 2018) Learning Objectives: 1. Utilize quantum numbers and atomic radii information to create input files and run a single-electron calculation. 2. Learn how to read the log and report files to obtain atomic orbital information. 3. Plot the all-electron wavefunction to determine where the electron is likely to be posi- tioned relative to the nucleus. Before doing this exercise, be sure to read through the Ins and Outs of Operation document. So, what do we need to build an atom? Protons, neutrons, and electrons of course! But the mass of a proton is 1800 times greater than that of an electron. Therefore, based on de Broglie’s wave equation, the wavelength of an electron is larger when compared to that of a proton. In other words, the wave-like properties of an electron are important whereas we think of protons and neutrons as particle-like. The separation of the electron from the nucleus is called the Born-Oppenheimer approximation. So now we need the wave-like description of the Hydrogen electron. Hydrogen is the simplest atom on the periodic table and the most abundant element in the universe, and therefore the perfect starting point for atomic orbitals and energies. The compu- tational tool we are going to use is called OPIUM (silly name, right?). Before we get started, we should know what’s needed to create an input file, which OPIUM calls a parameter files. Each parameter file consist of a sequence of ”keyblocks”, containing sets of related parameters. -

1.1. Introduction the Phenomenon of Positron Annihilation Spectroscopy
PRINCIPLES OF POSITRON ANNIHILATION Chapter-1 __________________________________________________________________________________________ 1.1. Introduction The phenomenon of positron annihilation spectroscopy (PAS) has been utilized as nuclear method to probe a variety of material properties as well as to research problems in solid state physics. The field of solid state investigation with positrons started in the early fifties, when it was recognized that information could be obtained about the properties of solids by studying the annihilation of a positron and an electron as given by Dumond et al. [1] and Bendetti and Roichings [2]. In particular, the discovery of the interaction of positrons with defects in crystal solids by Mckenize et al. [3] has given a strong impetus to a further elaboration of the PAS. Currently, PAS is amongst the best nuclear methods, and its most recent developments are documented in the proceedings of the latest positron annihilation conferences [4-8]. PAS is successfully applied for the investigation of electron characteristics and defect structures present in materials, magnetic structures of solids, plastic deformation at low and high temperature, and phase transformations in alloys, semiconductors, polymers, porous material, etc. Its applications extend from advanced problems of solid state physics and materials science to industrial use. It is also widely used in chemistry, biology, and medicine (e.g. locating tumors). As the process of measurement does not mostly influence the properties of the investigated sample, PAS is a non-destructive testing approach that allows the subsequent study of a sample by other methods. As experimental equipment for many applications, PAS is commercially produced and is relatively cheap, thus, increasingly more research laboratories are using PAS for basic research, diagnostics of machine parts working in hard conditions, and for characterization of high-tech materials. -

A Young Physicist's Guide to the Higgs Boson
A Young Physicist’s Guide to the Higgs Boson Tel Aviv University Future Scientists – CERN Tour Presented by Stephen Sekula Associate Professor of Experimental Particle Physics SMU, Dallas, TX Programme ● You have a problem in your theory: (why do you need the Higgs Particle?) ● How to Make a Higgs Particle (One-at-a-Time) ● How to See a Higgs Particle (Without fooling yourself too much) ● A View from the Shadows: What are the New Questions? (An Epilogue) Stephen J. Sekula - SMU 2/44 You Have a Problem in Your Theory Credit for the ideas/example in this section goes to Prof. Daniel Stolarski (Carleton University) The Usual Explanation Usual Statement: “You need the Higgs Particle to explain mass.” 2 F=ma F=G m1 m2 /r Most of the mass of matter lies in the nucleus of the atom, and most of the mass of the nucleus arises from “binding energy” - the strength of the force that holds particles together to form nuclei imparts mass-energy to the nucleus (ala E = mc2). Corrected Statement: “You need the Higgs Particle to explain fundamental mass.” (e.g. the electron’s mass) E2=m2 c4+ p2 c2→( p=0)→ E=mc2 Stephen J. Sekula - SMU 4/44 Yes, the Higgs is important for mass, but let’s try this... ● No doubt, the Higgs particle plays a role in fundamental mass (I will come back to this point) ● But, as students who’ve been exposed to introductory physics (mechanics, electricity and magnetism) and some modern physics topics (quantum mechanics and special relativity) you are more familiar with.. -

Lewis Structure Handout
Lewis Structure Handout for Chemistry Students An electron dot diagram, also known as a Lewis Structure, is a representation of valence electrons in a single atom, and can be further utilized to depict the bonds that form based on the available electrons for covalent bonding between multiple atoms. -Each atom has a characteristic dot diagram based on its position in the periodic table and this reflects its potential for fulfillment of the octet rule. As a general rule, the noble gases have a filled octet (column VIII with eight valence electrons) and each preceding column has successively one fewer. • For example, Carbon is in column IV and has four valence electrons. It can therefore be represented as: • Each of these "lone" electrons can form a bond by sharing the orbital with another unbonded electron. Hydrogen, being in column I, has a single valence electron, and will therefore satisfy carbon's octet thusly: • When a compound has bonded electrons (as with CH4) it is most accurate to diagram it with lines representing each of the single bonds. • Using carbon again for a slightly more complicated example, it can also form double bonds. This is the case when carbon bonds to two oxygen atoms, resulting in CO2. The oxygen atoms (with six valence electrons in column VI) are each represented as: • Because carbon is the least electronegative of the atoms involved, it is placed centrally, and its valence electrons will migrate toward the more electronegative oxygens to form two double bonds and a linear structure. This can be written as: where oxygen's remaining lone pairs are still shown. -

1 Solving Schrödinger Equation for Three-Electron Quantum Systems
Solving Schrödinger Equation for Three-Electron Quantum Systems by the Use of The Hyperspherical Function Method Lia Leon Margolin 1 , Shalva Tsiklauri 2 1 Marymount Manhattan College, New York, NY 2 New York City College of Technology, CUNY, Brooklyn, NY, Abstract A new model-independent approach for the description of three electron quantum dots in two dimensional space is developed. The Schrödinger equation for three electrons interacting by the logarithmic potential is solved by the use of the Hyperspherical Function Method (HFM). Wave functions are expanded in a complete set of three body hyperspherical functions. The center of mass of the system and relative motion of electrons are separated. Good convergence for the ground state energy in the number of included harmonics is obtained. 1. Introduction One of the outstanding achievements of nanotechnology is construction of artificial atoms- a few-electron quantum dots in semiconductor materials. Quantum dots [1], artificial electron systems realizable in modern semiconductor structures, are ideal physical objects for studying effects of electron-electron correlations. Quantum dots may contain a few two-dimensional (2D) electrons moving in the plane z=0 in a lateral confinement potential V(x, y). Detailed theoretical study of physical properties of quantum-dot atoms, including the Fermi-liquid-Wigner molecule crossover in the ground state with growing strength of intra-dot Coulomb interaction attracted increasing interest [2-4]. Three and four electron dots have been studied by different variational methods [4-6] and some important results for the energy of states have been reported. In [7] quantum-dot Beryllium (N=4) as four Coulomb-interacting two dimensional electrons in a parabolic confinement was investigated. -

Introduction to NMR Spectroscopy of Proteins
A brief introduction to NMR spectroscopy of proteins By Flemming M. Poulsen 2002 1 Introduction Nuclear magnetic resonance, NMR, and X-ray crystallography are the only two methods that can be applied to the study of three-dimensional molecular structures of proteins at atomic resolution. NMR spectroscopy is the only method that allows the determination of three-dimensional structures of proteins molecules in the solution phase. In addition NMR spectroscopy is a very useful method for the study of kinetic reactions and properties of proteins at the atomic level. In contrast to most other methods NMR spectroscopy studies chemical properties by studying individual nuclei. This is the power of the methods but sometimes also the weakness. NMR spectroscopy can be applied to structure determination by routine NMR techniques for proteins in the size range between 5 and 25 kDa. For many proteins in this size range structure determination is relatively easy, however there are many examples of structure determinations of proteins, which have failed due to problems of aggregation and dynamics and reduced solubility. It is the purpose of these notes to introduce the reader to descriptions and applications of the methods of NMR spectroscopy most commonly applied in scientific studies of biological macromolecules, in particular proteins. The figures 1,2 and 11 are copied from “Multidímensional NMR in Liquids” by F.J.M de Ven (1995)Wiley-VCH The Figure13 and Table 1 have been copied from “NMR of Proteins and Nucleic acis” K. Wüthrich (1986) Wiley Interscience The Figures 20, 21 and 22 have been copied from J. -

Introduction to Molecular Orbital Theory
Chapter 2: Molecular Structure and Bonding Bonding Theories 1. VSEPR Theory 2. Valence Bond theory (with hybridization) 3. Molecular Orbital Theory ( with molecualr orbitals) To date, we have looked at three different theories of molecular boning. They are the VSEPR Theory (with Lewis Dot Structures), the Valence Bond theory (with hybridization) and Molecular Orbital Theory. A good theory should predict physical and chemical properties of the molecule such as shape, bond energy, bond length, and bond angles.Because arguments based on atomic orbitals focus on the bonds formed between valence electrons on an atom, they are often said to involve a valence-bond theory. The valence-bond model can't adequately explain the fact that some molecules contains two equivalent bonds with a bond order between that of a single bond and a double bond. The best it can do is suggest that these molecules are mixtures, or hybrids, of the two Lewis structures that can be written for these molecules. This problem, and many others, can be overcome by using a more sophisticated model of bonding based on molecular orbitals. Molecular orbital theory is more powerful than valence-bond theory because the orbitals reflect the geometry of the molecule to which they are applied. But this power carries a significant cost in terms of the ease with which the model can be visualized. One model does not describe all the properties of molecular bonds. Each model desribes a set of properties better than the others. The final test for any theory is experimental data. Introduction to Molecular Orbital Theory The Molecular Orbital Theory does a good job of predicting elctronic spectra and paramagnetism, when VSEPR and the V-B Theories don't. -
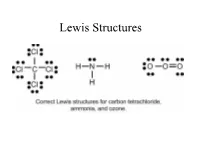
Lewis Structures
Lewis Structures Valence electrons for Elements Recall that the valence electrons for the elements can be determined based on the elements position on the periodic table. Lewis Dot Symbol Valence electrons and number of bonds Number of bonds elements prefers depending on the number of valence electrons. In general - F a m i l y → # C o v a l e n t B o n d s* H a l o g e n s X F , B r , C l , I → 1 bond often C a l c o g e n s O 2 bond often O , S → N i t r o g e n N 3 bond often N , P → C a r b o n C → 4 bond always C , S i The above chart is a guide on the number of bonds formed by these atoms. Lewis Structure, Octet Rule Guidelines When compounds are formed they tend to follow the Octet Rule. Octet Rule: Atoms will share electrons (e-) until it is surrounded by eight valence electrons. 4 unpaired 3unpaired 2unpaired 1unpaired up = unpaired e- 4 bonds 3 bonds 2 bonds 1 bond O=C=O N≡ N O = O F - F Atomic Connectivity The atomic arrangement for a molecule is usually given. CH2ClF HNO3 CH3COOH H2SO4 Cl N H O O O O H C F O H C C H O S O H H H H O H O In general when there is a single central atom in the 3- molecule, CH2ClF, SeCl2, O3 (CO2, NH3, PO4 ), the central atom is the first atom in the chemical formula.