Modeling 400 Million Years of Plant Hydraulics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Variation in Xylem Structure from Tropics to Tundra: Evidence from Vestured Pits
Variation in xylem structure from tropics to tundra: Evidence from vestured pits Steven Jansen*†, Pieter Baas‡, Peter Gasson§, Frederic Lens*, and Erik Smets* *Laboratory of Plant Systematics, Institute of Botany and Microbiology, Katholieke Universiteit Leuven, Kasteelpark Arenberg 31, B-3001 Leuven, Belgium; ‡Nationaal Herbarium Nederland, Universiteit Leiden Branch, P.O. Box 9514, 2300 RA Leiden, The Netherlands; and §Jodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3DS, United Kingdom Communicated by David L. Dilcher, University of Florida, Gainesville, FL, April 13, 2004 (received for review December 4, 2003) Bordered pits play an important role in permitting water flow among adjacent tracheary elements in flowering plants. Variation in the bordered pit structure is suggested to be adaptive in optimally balancing the conflict between hydraulic efficiency (con- ductivity) and safety from air entry at the pit membrane (air seeding). The possible function of vestured pits, which are bor- dered pits with protuberances from the secondary cell wall of the pit chamber, could be increased hydraulic resistance or minimized vulnerability to air seeding. These functional hypotheses have to be harmonized with the notion that the vestured or nonvestured nature of pits contains strong phylogenetic signals (i.e., often characterize large species-rich clades with broad ecological ranges). A literature survey of 11,843 species covering 6,428 genera from diverse climates indicates that the incidence of vestured pits considerably decreases from tropics to tundra. The highest fre- quencies of vestured pits occur in deserts and tropical seasonal woodlands. Moreover, a distinctly developed network of branched vestures is mainly restricted to warm habitats in both mesic and dry (sub)tropical lowlands, whereas vestures in woody plants from Fig. -

A Comparative Study of the Primary Vascular System Of
Amer. J. Bot. 55(4): 464-472. 1!16'>. A COMPARATIVE STUDY OF THE PRIMARY VASCULAR SYSTE~1 OF CONIFERS. III. STELAR EVOLUTION IN GYMNOSPERMS 1 KADAMBARI K. NAMBOODIRI2 AND CHARLES B. BECK Department of Botany, University of Michigan, Ann Arbor ABST RAe T This paper includes a survey of the nature of the primary vascular system in a large number of extinct gymnosperms and progymnosperms. The vascular system of a majority of these plants resembles closely that of living conifers, being characterized, except in the most primitive forms which are protostelic, by a eustele consisting of axial sympodial bundles from which leaf traces diverge. The vascular supply to a leaf originates as a single trace with very few exceptions. It is proposed that the eustele in the gyrr.nosperms has evolved directly from the protostele by gradual medullation and concurrent separation of the peripheral conducting tissue into longitudinal sympodial bundles from which traces diverge radially. A subsequent modification results in divergence of traces in a tangential plane, The closed vascular system of conifers with opposite and whorled phyllotaxis, in which the vascular supply to a leaf originates as two traces which subsequently fuse, is considered to be derived from the open sympodial system characteristic of most gymnosperms. This hypothesis of stelar evolution is at variance with that of Jeffrey which suggests that the eustele of seed plants is derived by the lengthening and overlapping of leaf gaps in a siphonostele followed by further reduction in the resultant vascular bundles. This study suggests strongly that the "leaf gap" of conifers and other extant gymnosperms is not homologous with that of siphonostelic ferns and strengthens the validity of the view that Pterop sida is an unnatural group. -

Lista Anotada De La Taxonomía Supraespecífica De Helechos De Guatemala Elaborada Por Jorge Jiménez
Documento suplementario Lista anotada de la taxonomía supraespecífica de helechos de Guatemala Elaborada por Jorge Jiménez. Junio de 2019. [email protected] Clase Equisetopsida C. Agardh α.. Subclase Equisetidae Warm. I. Órden Equisetales DC. ex Bercht. & J. Presl a. Familia Equisetaceae Michx. ex DC. 1. Equisetum L., tres especies, dos híbridos. β.. Subclase Ophioglossidae Klinge II. Órden Psilotales Prantl b. Familia Psilotaceae J.W. Griff. & Henfr. 2. Psilotum Sw., dos especies. III. Órden Ophioglossales Link c. Familia Ophioglossaceae Martinov c1. Subfamilia Ophioglossoideae C. Presl 3. Cheiroglossa C. Presl, una especie. 4. Ophioglossum L., cuatro especies. c2. Subfamilia Botrychioideae C. Presl 5. Botrychium Sw., tres especies. 6. Botrypus Michx., una especie. γ. Subclase Marattiidae Klinge IV. Órden Marattiales Link d. Familia Marattiaceae Kaulf. 7. Danaea Sm., tres especies. 8. Marattia Sw., cuatro especies. δ. Subclase Polypodiidae Cronquist, Takht. & W. Zimm. V. Órden Osmundales Link e. Familia Osmundaceae Martinov 9. Osmunda L., una especie. 10. Osmundastrum C. Presl, una especie. VI. Órden Hymenophyllales A.B. Frank f. Familia Hymenophyllaceae Mart. f1. Subfamilia Trichomanoideae C. Presl 11. Abrodictyum C. Presl, una especie. 12. Didymoglossum Desv., nueve especies. 13. Polyphlebium Copel., cuatro especies. 14. Trichomanes L., nueve especies. 15. Vandenboschia Copel., tres especies. f2. Subfamilia Hymenophylloideae Burnett 16. Hymenophyllum Sm., 23 especies. VII. Órden Gleicheniales Schimp. g. Familia Gleicheniaceae C. Presl 17. Dicranopteris Bernh., una especie. 18. Diplopterygium (Diels) Nakai, una especie. 19. Gleichenella Ching, una especie. 20. Sticherus C. Presl, cuatro especies. VIII. Órden Schizaeales Schimp. h. Familia Lygodiaceae M. Roem. 21. Lygodium Sw., tres especies. i. Familia Schizaeaceae Kaulf. 22. -

Agora Paleobotanica Un Hommage À / a Tribute to Bernard Renault (1836-1904)
Agora Paleobotanica Un hommage à / A tribute to Bernard Renault (1836-1904) 6-9/07/2015, Autun (France) Résumés - Abstracts Agora Paleobotanica Un hommage à / A tribute to Bernard Renault (1836-1904) 6-9/07/2015, Autun (France) Comité d’organisation / Organizing Committee Anais BOURA – Université Pierre et Marie Curie, Paris Jean BROUTIN – Université Pierre et Marie Curie, Paris Dominique CHABARD – Muséum d’Histoire Naturelle Jacques de La Comble, Autun Anne-Laure DECOMBEIX – CNRS-UMR AMAP, Montpellier Jean GALTIER – CNRS-UMR AMAP, Montpellier Georges GAND – Université de Bourgogne, Dijon Evelyne JONDOT– Muséum d’Histoire Naturelle Jacques de La Comble, Autun Brigitte MEYER-BERTHAUD – CNRS-UMR AMAP, Montpellier Progamme mis à jour le 29/06 Program updated on 29/06 Progamme mis à jour le 29/06 Program updated on 29/06 LUNDI/MONDAY Museum d’Histoire Naturelle Jacques de La Comble 14 rue Saint-Antoine, Autun. There is another access (backyard & garden) from Impasse Rollet. 16h00-17h30 Accueil des participants Participant arrival 17h30-18h Conférence introductive/ Opening talk Georges GAND. Le Bassin Permien d’Autun 18h-19h30 Allocution de bienvenue du maire - Apéritif de bienvenue Welcome address by the mayor - Welcome drinks Progamme mis à jour le 29/06 Program updated on 29/06 MARDI /TUESDAY Salon d’honneur de la mairie d’Autun/ City Hall Place du Champ de Mars. Session 1: PALEOZOÏQUE I Modérateurs/Chairs: Philippe GERRIENNE & Evelyn KUSTATSCHER 9h00-9h30 Jean GALTIER. Keynote: Bernard Renault (1836-1904), his life, works and paleobotanical heritage. 9h30-9h50 Christine STRULLU-DERRIEN & P. KENRICK. Palaeozoosporites renaultii, a new fungus in the rooting system of the Rhynie Chert plant Asteroxylon mackiei. -

<I> Salpichlaena</I>
Blumea 64, 2019: 1–22 www.ingentaconnect.com/content/nhn/blumea RESEARCH ARTICLE https://doi.org/10.3767/blumea.2018.64.01.01 Taxonomy and evolutionary history of the neotropical fern genus Salpichlaena (Blechnaceae) G.G. Cárdenas1,*, S. Lehtonen2, H. Tuomisto1 Key words Abstract Salpichlaena is a distinctive fern genus characterised by 2-pinnate climbing fronds with indeterminate growth. The number of species in the genus has been a matter of debate. Taxonomic studies are made difficult by ferns within-frond variability in pinna morphology and size, and by herbarium material being incomplete. We systematically hybrid documented 62 morphological traits in 283 herbarium specimens and sequenced 52 Salpichlaena and 11 outgroup Neotropics specimens. DNA sequences included plastid genes (rbcL, rpoC1 and rps4), intergenic spacers (rps4-trnS, trnH- phylogeny psbA and trnG-trnR) and a nuclear gene (pgiC). Phylogenetic analyses based on the plastid markers divided the Salpichlaena samples into six major clades. We recognise the three deepest clades as distinct species (S. hookeriana, S. papyrus systematics sp. nov. and S. volubilis), and each of the four shallower clades as a subspecies of S. volubilis. Furthermore, we taxonomy suggest that a group of specimens, placed into different clades in the plastid and nuclear trees and showing mixed morphological characters, represent a fourth species of hybrid origin (S. hybrida sp. nov.). The most important di- agnostic characters are: degree of lamina reduction in fertile pinnules; pinna/pinnule apex incisions, pinna/pinnule margin thickness and lamina texture in sterile pinna/pinnules; presence or absence of foliar buds; shape of scales; and the appearance of the abaxial surface of the lamina (uniform or with stomata on small white protuberances). -
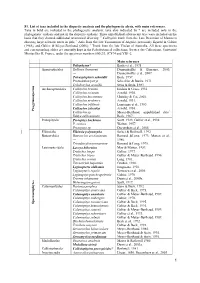
S1. List of Taxa Included in the Disparity Analysis and the Phylogenetic Alysis, with Main References
S1. List of taxa included in the disparity analysis and the phylogenetic alysis, with main references. Taxa in bold are included in the phylogenetic analysis; taxa also indicated by * are included only in the phylogenetic analysis and not in the disparity analysis. Three unpublished arborescent taxa were included on the basis that they showed additional anatomical diversity. 1 Callixylon trunk from the Late Devonian of Marrocco showing large sclerotic nests in pith; 2 Axis from the late Tournaisian of Algeria, previously figured in Galtier (1988), and Galtier & Meyer-Berthaud (2006); 3 Trunk from the late Viséan of Australia. All these specimens and corresponding slides are currently kept in the Paleobotanical collections, Service des Collections, Université Montpellier II, France, under the specimen numbers 600/2/3, JC874 and YB1-2. Main reference Psilophyton* Banks et al., 1975 Aneurophytales Rellimia thomsonii Dannenhoffer & Bonamo, 2003; --- Dannenhoffer et al., 2007. Tetraxylopteris schmidtii Beck, 1957. Proteokalon petryi Scheckler & Banks, 1971. Triloboxylon arnoldii Stein & Beck, 1983. s m Archaeopteridales Callixylon brownii Hoskin & Cross, 1951. r e Callixylon erianum Arnold, 1930. p s o Callixylon huronensis Chitaley & Cai, 2001. n Callixylon newberry Arnold, 1931. m y g Callixylon trifilievii Lemoigne et al., 1983. o r Callixylon zalesskyi Arnold, 1930. P Callixylon sp. Meyer-Berthaud, unpublished data1. Eddya sullivanensis Beck, 1967. Protopityales Protopitys buchiana Scott, 1923; Galtier et al., 1998. P. scotica Walton, 1957. Protopitys sp. Decombeix et al., 2005. Elkinsiales Elkinsia polymorpha Serbet & Rothwell, 1992. Buteoxylales Buteoxylon gordonianum Barnard &Long, 1973; Matten et al., --- 1980. Triradioxylon primaevum Barnard & Long, 1975. Lyginopteridales Laceya hibernica May & Matten, 1983. Tristichia longii Galtier, 1977. -

SYSTÉMATIQUE DES EMBRYOPHYTES Document Illustrant Plus Particulièrement Les Taxons Européens Version 4 Octobre 2007
Université Pierre et Marie Curie (PARIS 6) Préparation à l’agrégation SVSTU, secteur B SYSTÉMATIQUE DES EMBRYOPHYTES Document illustrant plus particulièrement les taxons européens version 4 octobre 2007 Catherine Reeb, Préparation agrégation SVSTU, UPMC Jean-Yves Dubuisson, Laboratoire Paléobotanique et Paléoécologie, équipe Paléodiversité, systématique et évolution des Embryophytes, UPMC Dessin de couverture : extrait d’un traité botanique tibétain Pour Garance Remerciements: merci à Jean et Monique Duperon pour les clés de détermination des familles d’Angiospermes, à A.M pour la reproduction de couverture, à Michaël Manuel et Eric Queinnec pour la partie I de l’introduction. Egalement à tous les illustrateurs qui ont autorisé l’utilisation de leurs documents mis en ligne sur Internet : Françoise Gantet, Prof. I. Foissner, Association Endemia de Nouvelle Calédonie. 1 Table des matières Introduction 3 Les données essentielles pour l’agrégation . 5 I Systématique générale des Embryophytes 6 I.1 Caractères des Embryophytes . 6 I.2 La place des Embryophytes au sein de la lignée verte . 7 I.3 Le groupe frère des Embryophytes . 7 I.4 Relations au sein des Embryophytes :les points acquis aujourd’hui . 8 I.4.1 Marchantiophytes, Bryophytes, Anthocérotophytes à la base des Embryophytes . 8 I.4.2 Les Trachéophytes ou plantes vasculaires, un groupe monophylétique . 9 I.4.3 Les Spermatophytes ou plantes à ovules, un groupe monophylétique . 9 I.5 Quelques points encore en discussion . 11 I.5.1 Position et relations des trois clades basaux (Bryophytes, Marchantiophytes et Anthocérotophytes) 11 I.5.2 Relations au sein des Spermatophytes :l’hypothèse "Gnepine" . 13 II Les principaux clades d’Embryophytes 15 II.1 MARCHANTIOPHYTA:Les Marchantiophytes ou Hépatiques . -

Tansley Review Evolution of Development of Vascular Cambia and Secondary Growth
New Phytologist Review Tansley review Evolution of development of vascular cambia and secondary growth Author for correspondence: Rachel Spicer1 and Andrew Groover2 Andrew Groover 1The Rowland Institute at Harvard, Cambridge, MA, USA; 2Institute of Forest Genetics, Pacific Tel: +1 530 759 1738 Email: [email protected] Southwest Research Station, USDA Forest Service, Davis, CA, USA Received: 29 December 2009 Accepted: 14 February 2010 Contents Summary 577 V. Evolution of development approaches for the study 587 of secondary vascular growth I. Introduction 577 VI. Conclusions 589 II. Generalized function of vascular cambia and their 578 developmental and evolutionary origins Acknowledgements 589 III. Variation in secondary vascular growth in angiosperms 581 References 589 IV. Genes and mechanisms regulating secondary vascular 584 growth and their evolutionary origins Summary New Phytologist (2010) 186: 577–592 Secondary growth from vascular cambia results in radial, woody growth of stems. doi: 10.1111/j.1469-8137.2010.03236.x The innovation of secondary vascular development during plant evolution allowed the production of novel plant forms ranging from massive forest trees to flexible, Key words: forest trees, genomics, Populus, woody lianas. We present examples of the extensive phylogenetic variation in sec- wood anatomy, wood formation. ondary vascular growth and discuss current knowledge of genes that regulate the development of vascular cambia and woody tissues. From these foundations, we propose strategies for genomics-based research in the evolution of development, which is a next logical step in the study of secondary growth. I. Introduction this pattern characterizes most extant forest trees, significant variation exists among taxa, ranging from extinct woody Secondary vascular growth provides a means of radially lycopods and horsetails with unifacial cambia (Cichan & thickening and strengthening plant axes initiated during Taylor, 1990; Willis & McElwain, 2002), to angiosperms primary, or apical growth. -

Devonian Plant Fossils a Window Into the Past
EPPC 2018 Sponsors Academic Partners PROGRAM & ABSTRACTS ACKNOWLEDGMENTS Scientific Committee: Zhe-kun Zhou Angelica Feurdean Jenny McElwain, Chair Tao Su Walter Finsinger Fraser Mitchell Lutz Kunzmann Graciela Gil Romera Paddy Orr Lisa Boucher Lyudmila Shumilovskikh Geoffrey Clayton Elizabeth Wheeler Walter Finsinger Matthew Parkes Evelyn Kustatscher Eniko Magyari Colin Kelleher Niall W. Paterson Konstantinos Panagiotopoulos Benjamin Bomfleur Benjamin Dietre Convenors: Matthew Pound Fabienne Marret-Davies Marco Vecoli Ulrich Salzmann Havandanda Ombashi Charles Wellman Wolfram M. Kürschner Jiri Kvacek Reed Wicander Heather Pardoe Ruth Stockey Hartmut Jäger Christopher Cleal Dieter Uhl Ellen Stolle Jiri Kvacek Maria Barbacka José Bienvenido Diez Ferrer Borja Cascales-Miñana Hans Kerp Friðgeir Grímsson José B. Diez Patricia Ryberg Christa-Charlotte Hofmann Xin Wang Dimitrios Velitzelos Reinhard Zetter Charilaos Yiotis Peta Hayes Jean Nicolas Haas Joseph D. White Fraser Mitchell Benjamin Dietre Jennifer C. McElwain Jenny McElwain Marie-José Gaillard Paul Kenrick Furong Li Christine Strullu-Derrien Graphic and Website Design: Ralph Fyfe Chris Berry Peter Lang Irina Delusina Margaret E. Collinson Tiiu Koff Andrew C. Scott Linnean Society Award Selection Panel: Elena Severova Barry Lomax Wuu Kuang Soh Carla J. Harper Phillip Jardine Eamon haughey Michael Krings Daniela Festi Amanda Porter Gar Rothwell Keith Bennett Kamila Kwasniewska Cindy V. Looy William Fletcher Claire M. Belcher Alistair Seddon Conference Organization: Jonathan P. Wilson -

Additional Observations on Zosterophyllum Yunnanicum Hsü from the Lower Devonian of Yunnan, China
This is an Open Access document downloaded from ORCA, Cardiff University's institutional repository: http://orca.cf.ac.uk/77818/ This is the author’s version of a work that was submitted to / accepted for publication. Citation for final published version: Edwards, Dianne, Yang, Nan, Hueber, Francis M. and Li, Cheng-Sen 2015. Additional observations on Zosterophyllum yunnanicum Hsü from the Lower Devonian of Yunnan, China. Review of Palaeobotany and Palynology 221 , pp. 220-229. 10.1016/j.revpalbo.2015.03.007 file Publishers page: http://dx.doi.org/10.1016/j.revpalbo.2015.03.007 <http://dx.doi.org/10.1016/j.revpalbo.2015.03.007> Please note: Changes made as a result of publishing processes such as copy-editing, formatting and page numbers may not be reflected in this version. For the definitive version of this publication, please refer to the published source. You are advised to consult the publisher’s version if you wish to cite this paper. This version is being made available in accordance with publisher policies. See http://orca.cf.ac.uk/policies.html for usage policies. Copyright and moral rights for publications made available in ORCA are retained by the copyright holders. @’ Additional observations on Zosterophyllum yunnanicum Hsü from the Lower Devonian of Yunnan, China Dianne Edwardsa, Nan Yangb, Francis M. Hueberc, Cheng-Sen Lib a*School of Earth and Ocean Sciences, Cardiff University, Park Place, Cardiff CF10 3AT, UK b Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China cNational Museum of Natural History, Smithsonian Institution, Washington D.C. 20560-0121, USA * Corresponding author, Tel.: +44 29208742564, Fax.: +44 2920874326 E-mail address: [email protected] ABSTRACT Investigation of unfigured specimens in the original collection of Zosterophyllum yunnanicum Hsü 1966 from the Lower Devonian (upper Pragian to basal Emsian) Xujiachong Formation, Qujing District, Yunnan, China has provided further data on both sporangial and stem anatomy. -

FOLIAR MORPHOLOGY and ANATOMY of the GIGANTOPTERID PLANT DELNORTEA ABBOTTIAE, from the LOWER PERMIAN of WEST Texasl
Arner. J. Bot. 75(9): 1409-1433. 1988. FOLIAR MORPHOLOGY AND ANATOMY OF THE GIGANTOPTERID PLANT DELNORTEA ABBOTTIAE, FROM THE LOWER PERMIAN OF WEST TEXASl SERGIUS H. MAMAY,2 JOHN M. MILLER,3 DAVID M. ROHR,4 AND WILLIAM E. STEIN, JR.5 'Department of Paleobiology, Smithsonian Institution, Washington, DC 20560; 'Department of Botany, Oregon State University, Corvallis, Oregon 97331; 4Department ofGeology, SuI Ross State University, Alpine, Texas 79832; and 'Museum of Paleontology, University of Michigan, Ann Arbor, Michigan 48109 ABSTRACT Delnortea is a monotypic genus (type-species: D. abbottiae) ofLower Permian gymnosperms based on leaves from uppermost Leonardian deltaic sediments exposed in the Del Norte Moun tains, West Texas. The leaves are simple, symmetrical, mostly oblong or elliptical, and vary in length from 1.2 to about 35 ern. The petioles are short and stout, with a basally enlarged abscission zone. The margins are crenate, with a narrow, indurated border. Venation is in 4 orders: the secondaries and tertiaries are robust, unbranched, and pinnately arranged in a precise "herringbone" pattern, with the secondaries ending in the marginal sinuses; the quaternaries divide sparingly and fuse with others to form a dense reticulum ofsmall meshes. Permineralized petiole and midrib material reflects a bifacial cambium, shown by a semicircular vascular arc, irregularly divided into several collateral bundles with secondary xylem and phloem. Delnortea is referable to the Gigantopteridaceae, a probably artificial family of gymnosperms incertae sedis with important venation features in common, but without known diagnostic reproductive organs. With Delnortea. the North American gigantopterids now include 5 genera, but Gigan topteris itselfis lacking. -

System Klasyfikacji Organizmów
1 SYSTEM KLASYFIKACJI ORGANIZMÓW Nie ma dziś ogólnie przyjętego systemu klasyfikacji organizmów. Nie udaje się osiągnąć zgodności nie tylko w odniesieniu do zakresów i rang poszczególnych taksonów, ale nawet co do zasad metodologicznych, na których klasyfikacja powinna być oparta. Zespołowe dzieła przeglądowe z reguły prezentują odmienne i wzajemnie sprzeczne podejścia poszczególnych autorów, bez nadziei na consensus. Nie ma więc mowy o skompilowaniu jednolitego schematu klasyfikacji z literatury i system przedstawiony poniżej nie daje nadziei na zaakceptowanie przez kogokolwiek poza kompilatorem. Przygotowany został w oparciu o kilka zasad (skądinąd bardzo kontrowersyjnych): (1) Identyfikowanie ga- tunków i ich klasyfikowanie w jednostki rodzajowe, rodzinowe czy rzędy jest zadaniem specjalistów i bez szcze- gółowych samodzielnych studiów nie można kwestionować wyników takich badań. (2) Podział świata żywego na królestwa, typy, gromady i rzędy jest natomiast domeną ewolucjonistów i dydaktyków. Powody, które posłużyły do wydzielenia jednostek powinny być jasno przedstawialne i zrozumiałe również dla niespecjalistów, albowiem (3) podstawowym zadaniem systematyki jest ułatwianie laikom i początkującym badaczom poruszanie się w obezwładniającej złożoności świata żywego. Wątpliwe jednak, by wystarczyło to do stworzenia zadowalającej klasyfikacji. W takiej sytuacji można jedynie przypomnieć, że lepszy ułomny system, niż żaden. Królestwo PROKARYOTA Chatton, 1938 DNA wyłącznie w postaci kolistej (genoforów), transkrypcja nie rozdzielona przestrzennie od translacji – rybosomy w tym samym przedzia- le komórki, co DNA. Oddział CYANOPHYTA Smith, 1938 (Myxophyta Cohn, 1875, Cyanobacteria Stanier, 1973) Stosunkowo duże komórki, dwuwarstwowa błona (Gram-ujemne), wewnętrzna warstwa mureinowa. Klasa CYANOPHYCEAE Sachs, 1874 Rząd Stigonematales Geitler, 1925; zigen – dziś Chlorofil a na pojedynczych tylakoidach. Nitkowate, rozgałęziające się, cytoplazmatyczne połączenia mię- Rząd Chroococcales Wettstein, 1924; 2,1 Ga – dziś dzy komórkami, miewają heterocysty.