Bird Species Richness in High-Andean Forest Fragments: Habitat Quality and Topography Matter
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Giant Hummingbird from Paramo De Chingaza, Colombia.-On 10 October 1981, During an Ornithological Survey at 3250 M Elev
GENERAL NOTES 661 E. nigrivestis is timed to coincide with flowering of P. huigrensis (and other plants) which bloom seasonally at lower elevations. The general ecology of E. nigrivestis is similar to that recorded for the closely related species, the Glowing Puffleg (E. vextitus)(Snow and Snow 1980). Snow and Snow (1980) observed E. vestitus feeding principally at plants of similar general morphology (straight tubular corollas 10-20 mm in length) in similar taxonomic groups (Cnvenclishia; Ericaceae more often than Palicourea; Rubiaceae for E. vestitus). Territoriality in E. vestitus was pronounced while this was only suggested in the male E. nigrivestis we observed, perhaps a seasonal effect. Finally E. vestitus, like E. nigrivestis, did not occur in tall forested habitats at similar elevations (Snow and Snow [1980] study at 2400-2500 m). The evidence presented here suggests that the greatly restricted range of E. nigrivestis is not due to dietary specializations. Male nectar sources are from a broad spectrum of plants, and the principal food plant of both sexes, P. huigrensis, is a widespread Andean species. Nesting requirements of E. nigrivestis may still be threatened with extinction through habitat destruction, especially if the species requires specific habitats of natural vegetation at certain times of year, such as that found on nude ridge crests. The vegetation on nudes in particular is rapidly disappearing because nudes provide flat ground for cultivation in otherwise pre- cipitous terrain. In light of its restricted range and the threat of habitat destruction resulting from such close proximity to a major urban center, Quito, we consider E. -

Northwest Argentina (Custom Tour) 13 – 24 November, 2015 Tour Leader: Andrés Vásquez Co-Guided by Sam Woods
Northwest Argentina (custom tour) 13 – 24 November, 2015 Tour leader: Andrés Vásquez Co-guided by Sam Woods Trip Report by Andrés Vásquez; most photos by Sam Woods, a few by Andrés V. Elegant Crested-Tinamou at Los Cardones NP near Cachi; photo by Sam Woods Introduction: Northwest Argentina is an incredible place and a wonderful birding destination. It is one of those locations you feel like you are crossing through Wonderland when you drive along some of the most beautiful landscapes in South America adorned by dramatic rock formations and deep-blue lakes. So you want to stop every few kilometers to take pictures and when you look at those shots in your camera you know it will never capture the incredible landscape and the breathtaking feeling that you had during that moment. Then you realize it will be impossible to explain to your relatives once at home how sensational the trip was, so you breathe deeply and just enjoy the moment without caring about any other thing in life. This trip combines a large amount of quite contrasting environments and ecosystems, from the lush humid Yungas cloud forest to dry high Altiplano and Puna, stopping at various lakes and wetlands on various altitudes and ending on the drier upper Chaco forest. Tropical Birding Tours Northwest Argentina, Nov.2015 p.1 Sam recording memories near Tres Cruces, Jujuy; photo by Andrés V. All this is combined with some very special birds, several endemic to Argentina and many restricted to the high Andes of central South America. Highlights for this trip included Red-throated -
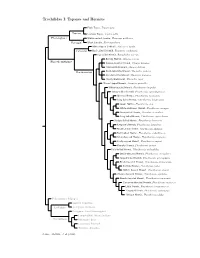
Topazes and Hermits
Trochilidae I: Topazes and Hermits Fiery Topaz, Topaza pyra Topazini Crimson Topaz, Topaza pella Florisuginae White-necked Jacobin, Florisuga mellivora Florisugini Black Jacobin, Florisuga fusca White-tipped Sicklebill, Eutoxeres aquila Eutoxerini Buff-tailed Sicklebill, Eutoxeres condamini Saw-billed Hermit, Ramphodon naevius Bronzy Hermit, Glaucis aeneus Phaethornithinae Rufous-breasted Hermit, Glaucis hirsutus ?Hook-billed Hermit, Glaucis dohrnii Threnetes ruckeri Phaethornithini Band-tailed Barbthroat, Pale-tailed Barbthroat, Threnetes leucurus ?Sooty Barbthroat, Threnetes niger ?Broad-tipped Hermit, Anopetia gounellei White-bearded Hermit, Phaethornis hispidus Tawny-bellied Hermit, Phaethornis syrmatophorus Mexican Hermit, Phaethornis mexicanus Long-billed Hermit, Phaethornis longirostris Green Hermit, Phaethornis guy White-whiskered Hermit, Phaethornis yaruqui Great-billed Hermit, Phaethornis malaris Long-tailed Hermit, Phaethornis superciliosus Straight-billed Hermit, Phaethornis bourcieri Koepcke’s Hermit, Phaethornis koepckeae Needle-billed Hermit, Phaethornis philippii Buff-bellied Hermit, Phaethornis subochraceus Scale-throated Hermit, Phaethornis eurynome Sooty-capped Hermit, Phaethornis augusti Planalto Hermit, Phaethornis pretrei Pale-bellied Hermit, Phaethornis anthophilus Stripe-throated Hermit, Phaethornis striigularis Gray-chinned Hermit, Phaethornis griseogularis Black-throated Hermit, Phaethornis atrimentalis Reddish Hermit, Phaethornis ruber ?White-browed Hermit, Phaethornis stuarti ?Dusky-throated Hermit, Phaethornis squalidus Streak-throated Hermit, Phaethornis rupurumii Cinnamon-throated Hermit, Phaethornis nattereri Little Hermit, Phaethornis longuemareus ?Tapajos Hermit, Phaethornis aethopygus ?Minute Hermit, Phaethornis idaliae Polytminae: Mangos Lesbiini: Coquettes Lesbiinae Coeligenini: Brilliants Patagonini: Giant Hummingbird Lampornithini: Mountain-Gems Tro chilinae Mellisugini: Bees Cynanthini: Emeralds Trochilini: Amazilias Source: McGuire et al. (2014).. -

TAG Operational Structure
PARROT TAXON ADVISORY GROUP (TAG) Regional Collection Plan 5th Edition 2020-2025 Sustainability of Parrot Populations in AZA Facilities ...................................................................... 1 Mission/Objectives/Strategies......................................................................................................... 2 TAG Operational Structure .............................................................................................................. 3 Steering Committee .................................................................................................................... 3 TAG Advisors ............................................................................................................................... 4 SSP Coordinators ......................................................................................................................... 5 Hot Topics: TAG Recommendations ................................................................................................ 8 Parrots as Ambassador Animals .................................................................................................. 9 Interactive Aviaries Housing Psittaciformes .............................................................................. 10 Private Aviculture ...................................................................................................................... 13 Communication ........................................................................................................................ -

Illegal Trade of the Psittacidae in Venezuela
Illegal trade of the Psittacidae in Venezuela A DA S ÁNCHEZ-MERCADO,MARIANNE A SMÜSSEN,JON P AUL R ODRÍGUEZ L ISANDRO M ORAN,ARLENE C ARDOZO-URDANETA and L ORENA I SABEL M ORALES Abstract Illegal wildlife trade is one of the major threats to trade involves avian species, poached to supply both domes- Neotropical psittacids, with nearly % of species targeted tic and international demand for pets (Rosen & Smith, for the illegal pet trade. We analysed the most comprehen- ). Among birds, Neotropical psittacids are of primary sive data set on illegal wildlife trade currently available for conservation concern, with nearly % of species affected Venezuela, from various sources, to provide a quantitative by poaching for the illegal pet trade (Olah et al., ). assessment of the magnitude, scope and detectability of The data used to measure the magnitude of the illegal pet the trade in psittacids at the national level. We calculated trade in psittacids have come from four main sources: seiz- a specific offer index (SO) based on the frequency of ure records and surveys of trappers (Cantú Guzmán et al., which each species was offered for sale. Forty-seven species ), literature reviews (Pires, ; Alves et al., ), dir- of psittacids were traded in Venezuela during –,of ect observation in markets (Herrera & Hennessey, ; which were non-native. At least , individuals were Gastañaga et al., ; Silva Regueira & Bernard, ), traded, with an overall extraction rate of , individuals and observation of the proportion of nest cavities poached per year ( years of accumulated reports). Amazona (Wright et al., ; Pain et al., ; Zager et al., ). ochrocephala was the most frequently detected species Each source has a unique geographical and taxonomic (SO = .), with the highest extraction rate (, indivi- coverage and evaluates different aspects of the market duals per year), followed by Eupsittula pertinax (SO = .) chain. -

Global Variation in Woodpecker Species Richness Shaped by Tree
Journal of Biogeography (J. Biogeogr.) (2017) 44, 1824–1835 ORIGINAL Global variation in woodpecker species ARTICLE richness shaped by tree availability † † Sigrid Kistrup Ilsøe1, , W. Daniel Kissling2, ,* , Jon Fjeldsa3, Brody Sandel4 and Jens-Christian Svenning1 1Section for Ecoinformatics and Biodiversity, ABSTRACT Department of Bioscience, Aarhus University, Aim Species richness patterns are generally thought to be determined by abi- DK-8000 Aarhus C, Denmark, 2Institute for otic variables at broad spatial scales, with biotic factors being only important Biodiversity and Ecosystem Dynamics (IBED), University of Amsterdam, 1090 GE at fine spatial scales. However, many organism groups depend intimately on Amsterdam, The Netherlands, 3Center for other organisms, raising questions about this generalization. As an example, Macroecology, Evolution and Climate at woodpeckers (Picidae) are closely associated with trees and woody habitats Natural History Museum of Denmark, because of multiple morphological and ecological specializations. In this study, University of Copenhagen, DK-1350 we test whether this strong biotic association causes woodpecker diversity to be Copenhagen K, Denmark, 4Department of closely linked to tree availability at a global scale. Biology, Santa Clara University, Santa Clara, Location Global. CA 95057, USA Methods We used spatial and non-spatial regressions to test for relationships between broad-scale woodpecker species richness and predictor variables describing current and deep-time availability of trees, current climate, Quater- nary climate change, human impact, topographical heterogeneity and biogeo- graphical region. We further used structural equation models to test for direct and indirect effects of predictor variables. Results There was a strong positive relationship between woodpecker species richness and current tree cover and annual precipitation, respectively. -
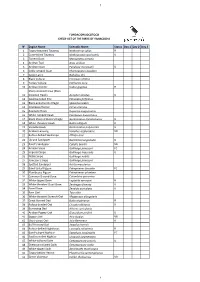
N° English Name Scientific Name Status Day 1
1 FUNDACIÓN JOCOTOCO CHECK-LIST OF THE BIRDS OF YANACOCHA N° English Name Scientific Name Status Day 1 Day 2 Day 3 1 Tawny-breasted Tinamou Nothocercus julius R 2 Curve-billed Tinamou Nothoprocta curvirostris U 3 Torrent Duck Merganetta armata 4 Andean Teal Anas andium 5 Andean Guan Penelope montagnii U 6 Sickle-winged Guan Chamaepetes goudotii 7 Cattle Egret Bubulcus ibis 8 Black Vulture Coragyps atratus 9 Turkey Vulture Cathartes aura 10 Andean Condor Vultur gryphus R Sharp-shinned Hawk (Plain- 11 breasted Hawk) Accipiter striatus U 12 Swallow-tailed Kite Elanoides forficatus 13 Black-and-chestnut Eagle Spizaetus isidori 14 Cinereous Harrier Circus cinereus 15 Roadside Hawk Rupornis magnirostris 16 White-rumped Hawk Parabuteo leucorrhous 17 Black-chested Buzzard-Eagle Geranoaetus melanoleucus U 18 White-throated Hawk Buteo albigula R 19 Variable Hawk Geranoaetus polyosoma U 20 Andean Lapwing Vanellus resplendens VR 21 Rufous-bellied Seedsnipe Attagis gayi 22 Upland Sandpiper Bartramia longicauda R 23 Baird's Sandpiper Calidris bairdii VR 24 Andean Snipe Gallinago jamesoni FC 25 Imperial Snipe Gallinago imperialis U 26 Noble Snipe Gallinago nobilis 27 Jameson's Snipe Gallinago jamesoni 28 Spotted Sandpiper Actitis macularius 29 Band-tailed Pigeon Patagoienas fasciata FC 30 Plumbeous Pigeon Patagioenas plumbea 31 Common Ground-Dove Columbina passerina 32 White-tipped Dove Leptotila verreauxi R 33 White-throated Quail-Dove Zentrygon frenata U 34 Eared Dove Zenaida auriculata U 35 Barn Owl Tyto alba 36 White-throated Screech-Owl Megascops -

Diversity and Natural History of Birds in Green Urban Areas of the City of Quito, Ecuador (America)
Diversity and natural history of birds in green urban areas of the city of Quito, Ecuador (America) Diego F. Cisneros-Heredia1,2,3* & Eliana Montenegro1 1 Universidad San Francisco de Quito, Colegio de Ciencias Biológicas y Ambientales, Laboratorio de Zoología Terrestre. Casilla Postal 17-1200-841, Quito, Ecuador 2 King’s College London, Department of Geography, Strand, London WC2R 2LS, UK 3 Aves&Conservación / BirdLife Ecuador, Quito, Ecuador * E-mail: [email protected] Abstract The diversity and ecology of urban bird communities have been extensively studied in Neartic and Paleartic areas, however, little is known about urban Neotropical areas. Quito, capital city of Ecuador, is located on a highland valley in the megadiverse tropical Andes. Founded in 1534, Quito did not increase significantly its urban area until the late 19th century, growing at an accelerated and unplanned rate during the 20th century. More than 100 species were known to inhabit in Quito at the end of the 19th century. Currently, most authors estimated that no more than 40 species occur in Quito, although no systematic bird studies have been conducted. Our research is a first approach to the avifauna of Quito, surveying the diversity living in green urban areas within the city borders. We used two field methodologies, i.e. line transects and point counts, to survey 16 green urban areas over 12 months. We recorded 65 species of birds, belonging to 20 families and 9 orders. Three species were the most common and frequent: Eared Dove Zenaida auriculata, Rufous-collared Sparrow Zonotrichia capensis, and Great Thrush Turdus fuscater, being omnivores and granivores adapted to anthropic habitats with low ecological complexity. -

Ecuador: the Andes & Mindo December 1
Ecuador: The Andes & Mindo December 1 – 9, 2016 Experience Ecuador’s Andean beauty and amazing bird diversity: from the hummingbirds of Yanacocha to the cloud forests of Bella Vista. Explore Antisana Volcano and search for endemics of the Chocó region; this trip is a must for those keen to explore South America. Visit the east and west sides of two branches of the Andes and bird key hotspots at Silanche, Milpe, Mindo, Guango, San Isidro, Papallacta Pass, and Antisana Volcano. Ecuador’s cloud forests host rarities like Highland Tinamou, Greater Scythebill, Bicolored Antbird, and the Sword-billed Hummingbird ― the only bird with a bill longer than its body. Savor delightful eco-lodges in forests lush with orchids, bromeliads, and butterflies, browse colorful markets, and enjoy warm Ecuadorian hospitality. Extend your trip to one of the Amazonia lodges if you choose. Tour Highlights Explore the important Yanacocha Reserve, with hummingbirds — including the amazing Sword-billed — as the star attraction Relax at the lovely Sachatamia Lodge, located on a private reserve; legendary birding is just out your door Bird a private farm, famous for views of the often difficult Giant Antpitta and Andean Cock-of-the-Rock Discover the abundant species of the lush cloud forest, 5,000 – 7,000 feet above sea level Trek the tundra-like high paramo and enjoy views of the stunning (and snow-capped) Antisana Volcano; our eyes are peeled for Andean Condor Bird and botanize in the cloud forests of San Isidro; 310 species abound Naturalist Journeys, LLC / Caligo Ventures PO Box 16545 Portal, AZ 85632 PH: 520.558.1146 / 800.426.7781 Fax 650.471.7667www.naturalistjourneys.com / www.caligo.com [email protected] / [email protected] Tour Summary 9-Day / 8-Night Birding & Natural History Tour with Expert Local Guides $2750 from Quito Airport is Mariscal Sucre International (UIO) Itinerary Thurs., Dec. -

A Case of Partial Albinism in Giant Conebill Oreomanes Fraseri
C o tin g a 1 8 A case of partial albinism in G iant C onebill O reom anes fraseri Grace P. Servat Cotinga 18 (2002): 80 Un individuo parcialmente albino del Pájaro de los Queñuales Oreomanes fraseri, fue observado y capturado en noviembre de 1997, en un bosque de Polylepis incana en la localidad de Japani, Lima, Perú. Este constituye el primer registro de albinismo para esta especie. Se presenta documentación fotográfica del individuo aberrante. Giant Conebill Oreomanes fraseri is a monotypic the Carnes Award from the American Ornitholo genus restricted to high-elevation Polylepis wood gists’ Union. lands, from south Colombia to south-west Bolivia3,5. Throughout the Andes of Peru O. fraseri is a con R e f e r e n c e s spicuous member of the highly threatened Polylepis 1. Arce, R. (1992) Distribución geográfica y avian community2. situación actual de los Polylepis en los I visited a Polylepis woodland in Japani (dpto. Departamentos de Ancash y Lima. Tesis. Lima, Huarochiri province, at 11°41'S 76°31'W and Universidad Nacional Agraria La Molina, 4140 m) from 28 November to 2 December 1997. A Lima. large (c. 300 ha) Polylepis incana woodland extends 2. Fjeldså, J. & Kessler, M. (1996) Conserving the along a chain of mountains, surrounded by bare ter biological diversity of Polylepis woodlands of rain, or dry shrubby vegetation1 (Fig. 1). On 30 the highlands of Peru and Bolivia. A contribu November, a partially albinistic O. fraseri was ob tion to sustainable natural resource manage served foraging on the bark of Polylepis trees, with ment in the high Andes. -

Dacninae Species Tree, Part I
Dacninae I: Nemosiini, Conirostrini, & Diglossini Hooded Tanager, Nemosia pileata Cherry-throated Tanager, Nemosia rourei Nemosiini Blue-backed Tanager, Cyanicterus cyanicterus White-capped Tanager, Sericossypha albocristata Scarlet-throated Tanager, Sericossypha loricata Bicolored Conebill, Conirostrum bicolor Pearly-breasted Conebill, Conirostrum margaritae Chestnut-vented Conebill, Conirostrum speciosum Conirostrini White-eared Conebill, Conirostrum leucogenys Capped Conebill, Conirostrum albifrons Giant Conebill, Conirostrum binghami Blue-backed Conebill, Conirostrum sitticolor White-browed Conebill, Conirostrum ferrugineiventre Tamarugo Conebill, Conirostrum tamarugense Rufous-browed Conebill, Conirostrum rufum Cinereous Conebill, Conirostrum cinereum Stripe-tailed Yellow-Finch, Pseudochloris citrina Gray-hooded Sierra Finch, Phrygilus gayi Patagonian Sierra Finch, Phrygilus patagonicus Peruvian Sierra Finch, Phrygilus punensis Black-hooded Sierra Finch, Phrygilus atriceps Gough Finch, Rowettia goughensis White-bridled Finch, Melanodera melanodera Yellow-bridled Finch, Melanodera xanthogramma Inaccessible Island Finch, Nesospiza acunhae Nightingale Island Finch, Nesospiza questi Wilkins’s Finch, Nesospiza wilkinsi Saffron Finch, Sicalis flaveola Grassland Yellow-Finch, Sicalis luteola Orange-fronted Yellow-Finch, Sicalis columbiana Sulphur-throated Finch, Sicalis taczanowskii Bright-rumped Yellow-Finch, Sicalis uropigyalis Citron-headed Yellow-Finch, Sicalis luteocephala Patagonian Yellow-Finch, Sicalis lebruni Greenish Yellow-Finch, -

Bolivia 2007 © Birdfinders 2007
Bolivia 7–25 September 2007 Participants: Didier Godreau Rolf Gräfvert Helge Grastveit Andrew Self Dennis and Margaret Weir Leader: Nick Acheson and Leo Catari (driver) Yellow-tufted Woodpecker Day 1 Overnight flight from London via Miami. Day 2 Having arrived smoothly courtesy of American Airlines, we immediately set to work in the savannahs surrounding the Viru Viru airport. Here we were delighted to see Greater Rhea, Red-winged Tinamou, Campo Flicker and flocks of Blue-crowned Parakeets. After a fine lunch in Santa Cruz we headed for the Piraí River on the west side of the city, and the Urubó savannahs beyond it. Once we found a sheltered spot out of the wind we had great birding, seeing, among many others, Speckled Chachalaca, Yellow-tufted Woodpecker, Blue-winged Parrotlet, Green-cheeked Parakeet, Golden-collared and Chestnut-fronted Macaws, Chestnut-eared Aracari, Thrush-like Wren, and Greater Thornbird. A pair of Titi Monkeys was also popular here. Day 3 This morning was spent at the Jardín Botánico, ten kilometres east of the city of Santa Cruz. By the roadside we saw White Woodpecker and Red-crested Cardinal and around the pond we found a dozy Brown-throated Three- toed Sloth, Social and Rusty-margined Flycatchers (very thoughtfully perched next to each other for ease of comparison), Blue-crowned Trogon, Blue-crowned Motmot and Narrow-billed Woodcreeper. Highlights in the forest included Rufous Casiornis, White-wedged Piculet, White-crested Tyrannulet, Fawn-breasted Wren, Ferruginous Pygmy-owl and a family of Silvery Marmosets. This afternoon we drove to Los Volcanes where we were greeted by Andean Condor, Military Macaw, Channel-billed Toucan, Red-billed and Turquoise-fronted Parrots and noisy, sky-filling flocks of Mitred Parakeets.