Audubon Flats Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

July 2020 Volume XCVI Number 7
July 2020 Volume XCVI Number 7 Commodore’s Reports Race Results Tennis Fleet New Members July • August 2020 SUNDAY MONDAY TUESDAY WEDNESDAY THURSDAY FRIDAY SATURDAY JULY 2 3 4 GALLEY WINDOW BAR RESUMES DECKHANDS LOCKER 1 HOURS NORMAL OPERATING HOURS (JULY 1) Contact Margaret Peebles Bulkhead Race Federal Holiday HAPPY 4th THURS & FRI 4-9p SAT 12-6p at (808) 342-1037 or email Mon-Fri Open 4p Dinghy Race SUNDAY 12-7p Sat Open 10a [email protected] 6p Sharp Start Sun Open 9a (Subject to Change) to make an appointment. 5 6 7 8 9 10 11 Deckhands Meeting 6p CG #14 6:30p- TBD Bulkhead Race Dinghy Race 6p Sharp Start ORF Singlehanded CG #17 6:30p - TBD 12 13 14 15 16 17 18 Classboat H Mooring 6p F & P 6:30p Bulkhead Race Dinghy Race IRF B-3 6p Sharp Start 19 20 21 22 23 24 25 Membership 6p Fleet Ops 6p Bulkhead Race Dinghy Race 6p Sharp Start JR Sailing Session 4 Begins 26 27 28 29 30 31 OFFICE HOURS WED-SUN Classboat B Bulkhead Race Dinghy Race 9a-4p BOD 7p 6p Sharp Start (Subject to Change) SUNDAY MONDAY TUESDAY WEDNESDAY THURSDAY FRIDAY SATURDAY August BAR HOURS OFFICE HOURS 1 WED-SUN Mon-Fri Open 4p 9a-4p Sat Open 10a Sun Open 9a (Subject to Change) 2 3 4 5 6 7 8 Deckhands Meeting 6p CG #14 6:30p- TBD Bulkhead Race Dinghy Race 6p Sharp Start CG #17 6:30p - TBD 9 10 11 12 13 14 15 Mooring 6p F & P 6:30p Bulkhead Race Dinghy Race 6p Sharp Start 16 17 18 19 20 21 22 Admissions Day Membership 6p Fleet Ops 6p Bulkhead Race Dinghy Race 6p Sharp Start 23 24 25 26 27 28 29 _____________________ ___________________ Bulkhead Race Dinghy Race 30 31 BOD 7p 6p Sharp Start RED = KYC Meeting BLUE = KYC Event / Racing GREEN = Deckhands Locker PURPLE= Holidays Black=Yoga /Revised Hours On the cover: Puanani at anchor in Waimea Bay. -

Herpetofaunal Inventories of the National Parks of South Florida and the Caribbean: Volume IV
Herpetofaunal Inventories of the National Parks of South Florida and the Caribbean: Volume IV. Biscayne National Park By Kenneth G. Rice1, J. Hardin Waddle1, Marquette E. Crockett 2, Christopher D. Bugbee2, Brian M. Jeffery 2, and H. Franklin Percival 3 1 U.S. Geological Survey, Florida Integrated Science Center 2 University of Florida, Department of Wildlife Ecology and Conservation 3 Florida Cooperative Fish and Wildlife Research Unit Open-File Report 2007-1057 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior DIRK KEMPTHORNE, Secretary U.S. Geological Survey Mark D. Myers, Director U.S. Geological Survey, Reston, Virginia: 2007 For product and ordering information: World Wide Web: http://www.usgs.gov/pubprod Telephone: 1-888-ASK-USGS Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this report is in the public domain, permission must be secured from the individual copyright owners to reproduce any copyrighted materials contained within this report. Suggested citation: Rice, K.G., Waddle, J.H., Crockett, M.E., Bugbee, C.D., Jeffery, B.M., and Percival, H.F., 2007, Herpetofaunal Inventories of the National Parks of South Florida and the Caribbean: Volume IV. Biscayne National Park: U.S. Geological Survey Open-File Report 2007-1057, 65 p. Online at: http://pubs.usgs.gov/ofr/2007/1057/ For more information about this report, contact: Dr. Kenneth G. Rice U.S. Geological Survey Florida Integrated Science Center UF-FLREC 3205 College Ave. Ft. Lauderdale, FL 33314 USA E-mail: [email protected] Phone: 954-577-6305 Fax: 954-577-6347 Dr. -

WMSDB - Worldwide Mollusc Species Data Base
WMSDB - Worldwide Mollusc Species Data Base Family: TURBINIDAE Author: Claudio Galli - [email protected] (updated 07/set/2015) Class: GASTROPODA --- Clade: VETIGASTROPODA-TROCHOIDEA ------ Family: TURBINIDAE Rafinesque, 1815 (Sea) - Alphabetic order - when first name is in bold the species has images Taxa=681, Genus=26, Subgenus=17, Species=203, Subspecies=23, Synonyms=411, Images=168 abyssorum , Bolma henica abyssorum M.M. Schepman, 1908 aculeata , Guildfordia aculeata S. Kosuge, 1979 aculeatus , Turbo aculeatus T. Allan, 1818 - syn of: Epitonium muricatum (A. Risso, 1826) acutangulus, Turbo acutangulus C. Linnaeus, 1758 acutus , Turbo acutus E. Donovan, 1804 - syn of: Turbonilla acuta (E. Donovan, 1804) aegyptius , Turbo aegyptius J.F. Gmelin, 1791 - syn of: Rubritrochus declivis (P. Forsskål in C. Niebuhr, 1775) aereus , Turbo aereus J. Adams, 1797 - syn of: Rissoa parva (E.M. Da Costa, 1778) aethiops , Turbo aethiops J.F. Gmelin, 1791 - syn of: Diloma aethiops (J.F. Gmelin, 1791) agonistes , Turbo agonistes W.H. Dall & W.H. Ochsner, 1928 - syn of: Turbo scitulus (W.H. Dall, 1919) albidus , Turbo albidus F. Kanmacher, 1798 - syn of: Graphis albida (F. Kanmacher, 1798) albocinctus , Turbo albocinctus J.H.F. Link, 1807 - syn of: Littorina saxatilis (A.G. Olivi, 1792) albofasciatus , Turbo albofasciatus L. Bozzetti, 1994 albofasciatus , Marmarostoma albofasciatus L. Bozzetti, 1994 - syn of: Turbo albofasciatus L. Bozzetti, 1994 albulus , Turbo albulus O. Fabricius, 1780 - syn of: Menestho albula (O. Fabricius, 1780) albus , Turbo albus J. Adams, 1797 - syn of: Rissoa parva (E.M. Da Costa, 1778) albus, Turbo albus T. Pennant, 1777 amabilis , Turbo amabilis H. Ozaki, 1954 - syn of: Bolma guttata (A. Adams, 1863) americanum , Lithopoma americanum (J.F. -

Another Successful Summer by Nick Mansbach T’S Been So Long Since I’Ve Written but the Clubs to Spend a Fun Filled Day on the Bay
COCONUT GROVE SAILING CLUB channelthe serving the community since 1945 OCTOBER 2008 Another Successful Summer by Nick Mansbach t’s been so long since I’ve written but the clubs to spend a fun filled day on the bay. At about sailing world has been busy, busy, busy! I’ll start 10am we loaded all the parents and all the kids by telling you about this year’s summer camp, on all different kinds of club boats; we had Prams, I Opti’s and Sunfish along with which was a tremendous success. This year we all our powerboats (including had a whopping 213 kids our good ‘ol Pontoon boat) and attending! The reason for headed out to the Dinner Key this dramatic increase was sandbar. If you’ve never seen a our staff; they were by Grandmother sailing in a Pram far the best I’ve ever had with their Granddaughter, let the opportunity to work me tell you it’s quite the sight! with, so thank you to all Once we arrived at the Sandbar the instructors and CIT’s everyone got an opportunity to (counselors in training) sail on all the different boats with who made this possible. their brothers, sisters, moms and We also had our second dads and instructors and CIT’s annual “Parent & kids fun day” which also turned as well. out to be a big hit. We started that morning with During our first hour there we were all treated to approximately 25 parents and about 40 kids looking something pretty cool, the Geico Racing Teams continued on page 8 COMMODORE’S REPORT opefully, by the time you get to read this, autumn will be starting to take hold, and the Hlong hot summer will just about be history. -

Coconut Grove Waterfront Master Plan
COCONUT GROVE WATERFRONT MASTER PLAN ERA / Curtis Rogers / ConsulTech / Paul George Ph.D. Agenda • City's Vision & Community Input • Framework Concepts • December 2006 Schemes • Draft Final Plan – Waterfront Open Space – Civic Core – Maritime Amenities & Facilities – Event Strategy – Roadway Strategy • Next Steps CITY'S VISION & COMMUNITY INPUT City's Vision & Requirements Vision for Coconut Grove's Waterfront • A coastal recreational park • Human scale • Public open space • Connectivity for the pedestrian realm • Waterfront promenades • Diverse open spaces • An active park • Sensitive environmental spoil island connections (real or visual) Requirements • A Plan that reflects the growth and desires of the community • An overhaul of the mooring fields to comply with the Federal Department of Environmental Protection • Spoil islands rehabilitation: cleaned of exotic plants, replanted with native species and redesigned for public access - Coconut Grove Waterfront & Spoil Islands Request for Qualifications Community Input 2004 Peacock Park Charrette • Lead by Friends of Peacock Park to develop a vision for the future of the Peacock Park • Charrette concepts: – Enhance landscaped open spaces – Minimal service parking only – Trim and "window" mangroves – Connection to spoil islands – Tie into local history – Redesign street frontage and articulate entrances – Redesign and seek alternative uses for Glass House – Outdoor cultural facility (amphitheater, waterfront plaza) – Hardcourts ok, no expansion Public Process • Stakeholder Input – May 2005 -

Flyer Jan10.Indd
In thIs Issue January C of C Regatta 1 MAC Wrap up 7 February President’s Column 2 Girls Rule 9 2•0•1•0 2010 Race Dates 4 Opposite Tack 10 MidWinters 5 Fleet 39 11 Helmsman 6 Classified 12 2009 ChampIonshIp of ChampIons A Publication of the American Y-Flyer Yacht Racing Association Regatta By Paul White Y-2782 Each year, a one-design sailboat is chosen to be raced in the Championship of Champions Regatta, also known as the C of C. This year, US Sailing Event Chairman, Drew Daugherty, selected the Lightning sailboat and asked the Carlyle Sailing Association to host the event. Twenty skippers, who are the reigning National, International, or North American Champions of their respective classes, are invited to compete. As the reigning Y-Flyer International Champion, I was invited to represent our class. The regatta was managed with precision by Drew Daugherty and Regatta Chairman, Matt Burridge, as well as a cadre of volunteers. The regatta began Wednesday morning with registration and a Lightening overview, including sailing tips, for my crew, Pat Passafiume and Steve Roeschlein, and myself. The remainder of Wednesday was spent honing our skills with several hours of practice racing and sailing. The afternoon practice races brought winds from the north in the low teens, white capping waters and air temperatures in the mid 40’s with a very cloudy and gray sky. The practice race course was approximately nine-tenths of a mile to windward, a mile to a leeward gate, and one- tenth of a mile upwind to the finish. -

Reproductive Development of Female Bonefish (Albula Spp.)
REPRODUCTIVE DEVELOPMENT OF FEMALE BONEFISH (ALBULA SPP.) FROM THE BAHAMAS by Cameron Alexander Luck A Thesis Submitted to the Faculty of The Charles E. Schmidt College of Science In Partial Fulfilment of the Requirements for the Degree of Master of Science Florida Atlantic University Boca Raton, Florida December 2018 Copyright 2018 by Cameron Luck ii ACKNOWLEDGEMENTS I sincerely appreciate the guidance of my advisor Dr. Matthew Ajemian for his continued support throughout this endeavor as well as the input from my committee members: Dr. Sahar Mejri, Dr. Aaron Adams, and Dr. Paul Wills. I am extremely grateful for the collaboration and support of Justin Lewis. Field collections would have been impossible without his local knowledge, willingness to be available, passion for bonefish, and incredible work ethic. I am also grateful for the words of wisdom and comedic relief often provided by fellow graduate students, lab members, and interns of the Fish Ecology and Conservation Lab: Grace Roskar, Breanna Degroot, Steve Lombardo, Rachel Shaw, Mike McCallister, Alex Allen, and many others. This project was financially supported by Bonefish & Tarpon Trust (BTT) and National Fish and Wildlife Foundation (NFWF). We are grateful for the lodging and water access provided by East End Lodge, Andros South, and Hank’s Place. The experimental protocol for this study received approval from Florida Atlantic University’s Institutional Animal Care and Use Committee (Animal Use Protocol #A16-34). iv ABSTRACT Author: Cameron Luck Title: Reproductive Development of Female Bonefish (Albula spp.) from the Bahamas Institution: Florida Atlantic University Thesis Advisor: Dr. Matthew J. Ajemian Degree: Master of Science Year: 2018 Bonefish (Albula spp.) support an economically important sport fishery, yet little is known regarding the reproductive biology of this genus. -

Florida Urban Forestry Council 2017 Issue Four
CouncilThe Quarterly Quarterly Newsletter of the Florida Urban Forestry Council 2017 Issue Four The Council Quarterly newsletter is published quarterly by the Florida Urban Forestry Council and is intended as an educational benefit to our members. Information may be reprinted if credit is given to the author(s) and this newsletter. All pictures, articles, advertisements, and other data are in no way to be construed as an endorsement of the author, products, services, or techniques. Likewise, the statements and opinions expressed herein are those of the individual authors and do not represent the view of the Florida Urban Forestry Council or its Executive Committee. This newsletter is made possible by the generous support of the Florida Department of Agriculture and Consumer Services, Florida Forest Service, Adam H. Putnam Commissioner. ADAPTIVE URBAN FOREST MANAGEMENT AND CATASTROPHIC WEATHER EVENTS: A CASE STUDY FROM EUGENE, OREGON Submitted by Scott Altenhoff and Eric Cariaga, City of Eugene, Oregon It seems our “new normal” is far There is, however, an upside to this story. and improvements through the frequent from normal; it’s time to expect the We have learned how to better address feedback. In the lines to follow, we’ll briefly catastrophic events. We use to plan and share the story of how Eugene made the unexpected and to do our best in base our work by looking back at past shift towards AM and how this shift made preparing for an uncertain future. models and tendencies. Now we practice a positive difference in both our storm a forward-looking approach. This new response and our overall operations. -
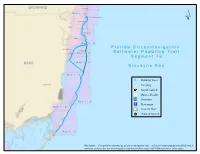
Segment 16 Map Book
Hollywood BROWARD Hallandale M aa p 44 -- B North Miami Beach North Miami Hialeah Miami Beach Miami M aa p 44 -- B South Miami F ll o r ii d a C ii r c u m n a v ii g a tt ii o n Key Biscayne Coral Gables M aa p 33 -- B S a ll tt w a tt e r P a d d ll ii n g T r a ii ll S e g m e n tt 1 6 DADE M aa p 33 -- A B ii s c a y n e B a y M aa p 22 -- B Drinking Water Homestead Camping Kayak Launch Shower Facility Restroom M aa p 22 -- A Restaurant M aa p 11 -- B Grocery Store Point of Interest M aa p 11 -- A Disclaimer: This guide is intended as an aid to navigation only. A Gobal Positioning System (GPS) unit is required, and persons are encouraged to supplement these maps with NOAA charts or other maps. Segment 16: Biscayne Bay Little Pumpkin Creek Map 1 B Pumpkin Key Card Point Little Angelfish Creek C A Snapper Point R Card Sound D 12 S O 6 U 3 N 6 6 18 D R Dispatch Creek D 12 Biscayne Bay Aquatic Preserve 3 ´ Ocean Reef Harbor 12 Wednesday Point 12 Card Point Cut 12 Card Bank 12 5 18 0 9 6 3 R C New Mahogany Hammock State Botanical Site 12 6 Cormorant Point Crocodile Lake CR- 905A 12 6 Key Largo Hammock Botanical State Park Mosquito Creek Crocodile Lake National Wildlife Refuge Dynamite Docks 3 6 18 6 North Key Largo 12 30 Steamboat Creek John Pennekamp Coral Reef State Park Carysfort Yacht Harbor 18 12 D R D 3 N U O S 12 D R A 12 C 18 Basin Hills Elizabeth, Point 3 12 12 12 0 0.5 1 2 Miles 3 6 12 12 3 12 6 12 Segment 16: Biscayne Bay 3 6 Map 1 A 12 12 3 6 ´ Thursday Point Largo Point 6 Mary, Point 12 D R 6 D N U 3 O S D R S A R C John Pennekamp Coral Reef State Park 5 18 3 12 B Garden Cove Campsite Snake Point Garden Cove Upper Sound Point 6 Sexton Cove 18 Rattlesnake Key Stellrecht Point Key Largo 3 Sound Point T A Y L 12 O 3 R 18 D Whitmore Bight Y R W H S A 18 E S Anglers Park R 18 E V O Willie, Point Largo Sound N: 25.1248 | W: -80.4042 op t[ D A I* R A John Pennekamp State Park A M 12 B N: 25.1730 | W: -80.3654 t[ O L 0 Radabo0b. -

Pseudophoenix Sargentii) on Elliott Key, Biscayne National Park
Status update: Long-term monitoring of Sargent’s cherry palm (Pseudophoenix sargentii) on Elliott Key, Biscayne National Park FEBRUARY 2021 Acknowledgments Thank you to Biscayne National Park for more than three decades of support for this collaborative project on Pseudophoenix sargentii augmentation and demographic monitoring. Monitoring in 2021 was funded by the Florida Dept. of Agriculture and Consumer Services, Division of Plant Industry, under Agreement #027132. For assistance with field work and logistics we would like to thank Morgan Wagner, Michael Hoffman, Brian Lockwood, Shelby Moneysmith, Vanessa McDonough, and Astrid Santini from Biscayne National Park, Eliza Gonzalez from Montgomery Botanical Center, Dallas Hazelton from Miami-Dade County, and volunteer biologists Joseph Montes de Oca and Elizabeth Wu. Thank you to the cooperators whose excellent past work on this project has laid the foundation upon which we stand today, especially Carol Lippincott, Rob Campbell, Joyce Maschinski, Janice Duquesnel, Dena Garvue, and Sam Wright. Janice Duquesnel provided important details about the 1991-1994 outplanting efforts. Suggested citation Possley, J., J. Lange, S. Wintergerst and L. Cuni. 2021. Status update: long-term monitoring of Pseudophoenix sargentii on Elliott Key, Biscayne National Park. Unpublished report from Fairchild Tropical Botanic Garden, funded by Florida Dept. of Agriculture and Consumer Services Agreement #027132. Background Pseudophoenix sargentii H.Wendl. ex Sarg., known by the common names ‘Sargent’s cherry palm’ and ‘buccaneer palm,’ is a slow-growing palm native to coastal habitats throughout the Caribbean basin, where it grows on exposed limestone or in humus or sand over limestone. Over the past century, the species has declined throughout its range, due to in part to overharvesting for use in landscaping. -

A BRIEF HISTORY of PINE FLATWOODS in SOUTH FLORIDA Submitted by Jerry Renick, Environmental Service Manager - Wantman Group, Inc
CouncilThe Quarterly Quarterly Newsletter of the Florida Urban Forestry Council 2016 Issue Three The Council Quarterly newsletter is published quarterly by the Florida Urban Forestry Council and is intended as an educational benefit to our members. Information may be reprinted if credit is given to the author(s) and this newsletter. All pictures, articles, advertisements, and other data are in no way to be construed as an endorsement of the author, products, services, or techniques. Likewise, the statements and opinions expressed herein are those of the individual authors and do not represent the view of the Florida Urban Forestry Council or its Executive Committee. This newsletter is made possible by the generous support of the Florida Department of Agriculture and Consumer Services, Florida Forest Service, Adam H. Putnam Commissioner. A BRIEF HISTORY OF PINE FLATWOODS IN SOUTH FLORIDA Submitted by Jerry Renick, Environmental Service Manager - Wantman Group, Inc. Pine flatwoods were first identified as pine extending into the Bahama Islands. This shrubs including saw palmetto, galberry, barrens by William Bartram in 1791 during south Florida variety of pine is also known lyonia, cocoplum, and a few other common his travels in Florida. The term flatwoods as yellow pine, swamp pine, and pitch species. They are usually inhabited with was used by the English settlers in the pine. It is estimated that pine flatwoods a diverse groundcover of grasses and early days to describe the characteristic flat as an ecosystem covered up to 50% of the wildflowers. The terrain for pine flatwoods ground with no topographic relief in south land area of Florida at one time. -

Florida Coral Reefs: Islandia
FOREWORD· In their present relatively undeveloped state, the upper Florida Keys and the adjoining waters and submerged. lands of Biscayne ~ay and the Atlantic Ocean are an enviromr~.ental element highly important to Florida ancl a valuable recreation resource for the nation. Fully aware that intensive private development would greatly alter . these values, the Secretary of the Interior directed the National Park Service and the Bureau of Outdoor Recreation, assisted by the Fish and Wildlife Service, to conduct studies of the area. This interim professional report is the r~sult of these studies. It is being distributed now to solicit the comments and suggestions of interested parties. The additional information obtained in this manner will be utilized by the National Park Service and the Bureau of Outdoor Recre ation to complete the study and formulate recommendations to the Secretary· of the Interior. It is requested that comments and suggestions on this interim profes sional report be sent to the Regional Director, Southeast Region, Na tional Park Service, P. 0. Box 10008, Richmond, Virginia 23240. Material should be submitted in time to reach the Regional Director on or before August 15, 1965. ~~ Edward C. Crafts Director Director Bureau of Outdoor Recreation National Park Service Page No. FOREWORD THE STUDY 2 THE CORAL REEFS The Resource 3 The Climate 5 The Ecology 6 MAN AND THE CORAL REEFS 10 THE SITUATION Development Possibilities · 12 Significance for Preservation 12 THE OPPORTUNITIES 18 Alternative Plans 14' Plan 1 16 Plan 2 20 Plan 3 24 THE STUDY In response to interest expressed by the weekending, and vacationing are engaged Dade Co1:-1nty Board of Commissioners and in extensively.