AMMONIUM NONANOATE (PC Code 031802) �
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pest Management Strategic Plan for Strawberry Production in California
PEST MANAGEMENT STRATEGIC PLAN FOR STRAWBERRY PRODUCTION IN CALIFORNIA 1 | Page TABLE OF CONTENTS INTRODUCTION ................................................................................................................................................... 5 Previous Pest Management Strategic Plan .............................................................................................. 5 Development of the New Plan .................................................................................................................... 5 STRAWBERRY MARKET SHARE AND VALUE.................................................................................................... 10 STRAWBERRY PRODUCTION OVERVIEW ........................................................................................................ 13 PEST MANAGEMENT FOR COMMERCIAL STRAWBERRY PLANTINGS ......................................................... 22 LAND PREPARATON THROUGH PLANTING ............................................................................................... 22 Activities Prior to Fumigation................................................................................................................. 22 Soil Fumigation ........................................................................................................................................ 22 Planting ..................................................................................................................................................... 25 Work Group Recommendations for -

Ammonium Nonanoate
1 2 Petition to 3 The National Organic Program and 4 National Organic Standards Board to 5 Add Ammonium Nonanoate to the 6 USDA - National Organic Standards - National List 7 8 9 10 11 Date: 12 13 September 27, 2016 14 15 16 17 18 Submitted by: 19 20 Emery Oleochemicals LLC 21 Agro Green Business 22 4900 Este Avenue 23 Cincinnati, Ohio 45232 24 USA 25 26 Phone: 513-762-2500 27 28 29 30 United States Department of Agriculture 31 Agricultural Marketing Service 32 National Organic Program 33 1400 Independence Avenue S.W. 34 Room 2642-South Building 35 Washington, D.C. 20250 36 37 Regarding: Petition to The National Organic Standards Board (NOSB) to Amend the National 38 List of Allowed and Prohibited Substances at 7 C.F.R. §§ 205.600-205.606. 39 40 We hereby petition the National Organic Standards Board to amend the National List (7 C.F.R. 41 §§ 205.600-205.606) to include ammonium nonanoate as a non-selective herbicide for use in 42 organic agricultural practices for production of food and fiber. Specifically, we propose that 43 ammonium nonanoate be added to 7 C.F.R. § 205.601 (Synthetic substances allowed for use in 44 organic crop production) of the National Organic Standards National List as follows: 45 46 7 C.F.R. § 205.601(b)(3) Ammonium nonanoate, a soap-based, non-selective weed control agent 47 for use in food and ornamental crops for control of weeds and crop desiccation as a harvest aid. 48 49 While a non-selective, synthetic substance petitioned for use as an herbicide has not been 50 approved to date by the NOSB, this petition will provide justification for that approval. -

New England Vegetable Management Guide 2020-2021 Edition.Pdf
New England Vegetable Management Guide 2020-2021 Edition Vegetable Crop Production from Seed to Harvest COOPERATIVE EXTENSION Cover photo credit: Farmers and Extension Educators scout onions at High Meadows Farm in Westminster, VT. Photo by: Vern Grubinger, 2014. We would like to acknowledge the support received from the USDA Risk Management Agency to assist with the revision and publication of this document. 2020-2021 New England Vegetable Management Guide GENERAL EDITOR: Katie Campbell-Nelson SECTION EDITORS: Katherine Ghantous, Weeds Becky Sideman, Cultural Practices Cheryl Smith, Diseases Anna Wallingford, Insects PRODUCTION MANAGER: Lisa McKeag CONTRIBUTORS: University of Connecticut Shuresh Ghimire, Cultural Practices Leanne Pundt, Vegetable Transplants University of Maine James Dwyer, Cultural Practices David T. Handley, Cultural Practices Mark Hutton, Cultural Practices Alicyn Smart, Diseases University of Massachusetts Katie Campbell-Nelson, Cultural Practices Genevieve Higgins, Cultural Practices Angela Madeiras, Diseases Lisa McKeag, Cultural Practices Maggie Ng, Pest Management Susan Scheufele, Cultural Practices Tom Smiarowski, Risk Management University of New Hampshire Heather Bryant, Cultural Practices Jeremy Delisle, Cultural Practices Alan Eaton, Vertebrate Pest Management George Hamilton, Cultural Practices Olivia Saunders, Cultural Practices Becky Sideman, Cultural Practices University of Rhode Island Andy Radin, Cultural Practices University of Vermont Vern Grubinger, Cultural Practices Ann Hazelrigg, Diseases Maine Organic Farmers and Gardeners Association Eric Sideman, Cultural Practices Caleb Goossen, Cultural Practices GRAPHIC DESIGN: Mickey Boisvert, MBDesign PAST CONTRIBUTORS: The following people have contributed to this publication over many years and this Guide reflects many of their contributions: Richard Ashley, A. Richard Bonnano, Ed Boutin, Jude Boucher, Kristen Castrataro, Pearly Colby, Douglas Cox, Jim Dill, Bess M. -

Www .Alfa.Com
Bio 2013-14 Alfa Aesar North America Alfa Aesar Korea Uni-Onward (International Sales Headquarters) 101-3701, Lotte Castle President 3F-2 93 Wenhau 1st Rd, Sec 1, 26 Parkridge Road O-Dong Linkou Shiang 244, Taipei County Ward Hill, MA 01835 USA 467, Gongduk-Dong, Mapo-Gu Taiwan Tel: 1-800-343-0660 or 1-978-521-6300 Seoul, 121-805, Korea Tel: 886-2-2600-0611 Fax: 1-978-521-6350 Tel: +82-2-3140-6000 Fax: 886-2-2600-0654 Email: [email protected] Fax: +82-2-3140-6002 Email: [email protected] Email: [email protected] Alfa Aesar United Kingdom Echo Chemical Co. Ltd Shore Road Alfa Aesar India 16, Gongyeh Rd, Lu-Chu Li Port of Heysham Industrial Park (Johnson Matthey Chemicals India Toufen, 351, Miaoli Heysham LA3 2XY Pvt. Ltd.) Taiwan England Kandlakoya Village Tel: 866-37-629988 Bio Chemicals for Life Tel: 0800-801812 or +44 (0)1524 850506 Medchal Mandal Email: [email protected] www.alfa.com Fax: +44 (0)1524 850608 R R District Email: [email protected] Hyderabad - 501401 Andhra Pradesh, India Including: Alfa Aesar Germany Tel: +91 40 6730 1234 Postbox 11 07 65 Fax: +91 40 6730 1230 Amino Acids and Derivatives 76057 Karlsruhe Email: [email protected] Buffers Germany Tel: 800 4566 4566 or Distributed By: Click Chemistry Reagents +49 (0)721 84007 280 Electrophoresis Reagents Fax: +49 (0)721 84007 300 Hydrus Chemical Inc. Email: [email protected] Uchikanda 3-Chome, Chiyoda-Ku Signal Transduction Reagents Tokyo 101-0047 Western Blot and ELISA Reagents Alfa Aesar France Japan 2 allée d’Oslo Tel: 03(3258)5031 ...and much more 67300 Schiltigheim Fax: 03(3258)6535 France Email: [email protected] Tel: 0800 03 51 47 or +33 (0)3 8862 2690 Fax: 0800 10 20 67 or OOO “REAKOR” +33 (0)3 8862 6864 Nagorny Proezd, 7 Email: [email protected] 117 105 Moscow Russia Alfa Aesar China Tel: +7 495 640 3427 Room 1509 Fax: +7 495 640 3427 ext 6 CBD International Building Email: [email protected] No. -
Index to FCC 11 the Following Index Is for Convenience and Informational Use Only and Shall Not Be Used for Interpretive Purposes
Index to FCC 11 The following Index is for convenience and informational use only and shall not be used for interpretive purposes. In addition to effective articles, this Index may also include items recently omitted from the FCC in the indicated Book or Supplement. The monographs and general tests and assay listed in this Index may reference other general test and assay specifications. The articles listed in this Index are not intended to be autonomous standards and should only be interpreted in the context of the entire FCC publication. For the most current version of the FCC please see the FCC Online. FCC 11 Index / Allyl Butanoate / I-1 Index Index Titles of monographs are shown in the boldface type. A 2-Acetyl Thiazole, 18 Alcohol C-8, 863 Acetyl Valeryl, 562 Alcohol C-9, 854 Abbreviations, 6 Acetyl Value, 1400 Alcohol C-10, 362 Absolute Alcohol (Reagent), 5 Achilleic Acid, 24 Alcohol C-11, 1231 Acacia, 556 Acid (Reagent), 5 Alcohol C-12, 681 ªAccuracyº, Defined, 1538 Acid-Hydrolyzed Milk Protein, 22 Alcohol C-16, 569 Acesulfame K, 9 Acid-Hydrolyzed Proteins, 22 Alcohol Content of Ethyl Oxyhydrate Acesulfame Potassium, 9 Acid Calcium Phosphate, 219 Flavor Chemicals (Other than Acetal, 10 Acid Hydrolysates of Proteins, 22 Essential Oils), 1437 Acetaldehyde, 10 Acidic Sodium Aluminum Phosphate, Alcohol, Diluted, 1524 Acetaldehyde Diethyl Acetal, 10 1065 Alcoholic Potassium Hydroxide TS, Acetaldehyde Test Paper, 1535 Acidified Sodium Chlorite 1524 Acetals (Essential Oils and Flavors), Solutions, 23 Alcoholometric Table, 1644 1395 Acidity -
WO 2018/164999 Al 13 September 2018 (13.09.2018) W !P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2018/164999 Al 13 September 2018 (13.09.2018) W !P O PCT (51) International Patent Classification: (74) Agent: MALLON, Joseph, J.; Knobbe Martens Olson & C0SG 3/02 (2006 .0 1) C0SD 9/02 (2006 .01) Bear LLP, 2040 Main Street, Fourteenth Floor, Irvine, CA A01N 59/14 (2006.01) 92614 (US). (21) International Application Number: (81) Designated States (unless otherwise indicated, for every PCT/US20 18/020868 kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, (22) International Filing Date: CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, 05 March 2018 (05.03.2018) DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, (25) Filing Language: English HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, (26) Publication Language: English MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (30) Priority Data: OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, 62/469,087 09 March 20 17 (09.03 .20 17) US SC, SD, SE, SG, SK, SL, SM, ST, SV, SY,TH, TJ, TM, TN, 62/609,137 2 1 December 20 17 (2 1. 12.20 17) US TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Better Product, Greener Results Table of Contents
BETTER PRODUCT, GREENER RESULTS TABLE OF CONTENTS GreenYard® Premium Turfgrass Seeds .................................... 2 & 3 GreenYard® Turfgrass Mixes Use Chart. 4 & 5 Grass Seeds ......................................................... 6 & 7 Grass Seed Characteristics .................................................8 Greens Grade Fertilizers (SGN 100) ..........................................9 Professional Standard Grade Fertilizers .....................................10 Turf Fertilizers with Preemergence Herbicides ................................11 Turf Fertilizers with Postemergence Herbicides ................................12 Turf Professional Fertilizers with Insecticide ..................................12 Straight Grade Fertilizers .................................................13 Organic and Organic Blend Fertilizers .......................................14 Liquid Fertilizers ........................................................15 Soluble Fertilizers .......................................................15 Athletic Field Products ...................................................17 Erosion Control Products .............................................. 18-21 Herbicides .......................................................... 22-30 Insecticides ......................................................... 31-32 Fungicides .......................................................... 33-36 Industrial, Bareground & Right-of-Way .................................... 37-40 Adjuvants, Surfactants, and Colorants ................................... -

Ammonium Nonanoate
Ammonium Nonanoate Crops 1 2 Identification of Petitioned Substance 3 4 Chemical Name: 5 Octane-1-carboxylic acid, ammonium salt CAS Number: 6 63718-65-0 (ammonium nonanoate) 7 Other Names: 8 Ammonium pelargonate Other Codes: 9 Pelargonic acid, ammonium salt 031802 (EPA PC code for ammonium nonanoate; 10 Nonanoic acid, ammonium salt EPA, 2008) 11 031801 (EPA PC code for ammonium salts of C8- 12 Trade Names: C18 and C18’ fatty acids) 13 FL-AN140F (formerly Racer® Concentrate) 14 FL-AN405F (formerly Racer® Ready to Use) 15 16 Characterization of Petitioned Substance 17 18 Composition of the Substance: 19 Ammonium nonanoate is a C9 saturated-chain fatty acid soap salt with the chemical formula NH4(C9H18O2). 20 Ammonium salts of fatty acids, including ammonium nonanoate, are mineral salts of naturally-occurring fatty 21 acids in the environment (75 FR 14082). Ammonium nonanoate is the ammonium salt of nonanoic acid (CAS No. 22 112-05-0). Nonanoic acid, also known as pelargonic acid, is found in almost all species of animals and plants and 23 is present at low levels in many commonly eaten foods (EPA, 2000). The molecular structure of ammonium 24 nonanoate is shown in Figure 1. 25 26 Figure 1. Molecular Structure of Ammonium Nonanoate 27 28 29 Source: created using MarvinSketch software 30 available on ChemIDplus Advanced (2011) 31 32 33 Properties of the Substance: 34 Ammonium nonanoate is produced as a flowable concentrate for use in agriculture and as a ready-to-use 35 solution for use in residential and landscaping settings. The product for agricultural uses that is registered 36 by the petitioner (Falcon Labs, LLC) is named Racer® Concentrate (currently listed as “FL-AN140F” in the 37 National Pesticide Information Retrieval System [NPIRS]). -

Ionic Liquids Comprising Nitrogen Containing Cations
(19) & (11) EP 2 322 497 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 18.05.2011 Bulletin 2011/20 C07C 215/40 (2006.01) B01J 31/04 (2006.01) B01J 31/02 (2006.01) (21) Application number: 10181447.3 (22) Date of filing: 07.04.2005 (84) Designated Contracting States: (72) Inventor: Walker, Adam, John AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Littleport, Cambridgeshire CB6 1HJ (GB) HU IE IS IT LI LT LU MC NL PL PT RO SE SI SK TR (74) Representative: Brand, Thomas Louis (30) Priority: 07.04.2004 GB 0407908 W.P. Thompson & Co. 55 Drury Lane (62) Document number(s) of the earlier application(s) in London WC2B 5SQ (GB) accordance with Art. 76 EPC: 05735988.7 / 1 805 131 Remarks: This application was filed on 28-09-2010 as a (71) Applicant: Innovia Films Limited divisional application to the application mentioned Wigton under INID code 62. Cumbria CA7 9BG (GB) (54) Ionic liquids comprising nitrogen containing cations (57) The present invention relates to ionic liquid comprising an anion and a cation wherein the cation is a primary, secondary or tertiary ammonium ion containing a protonated nitrogen atom. EP 2 322 497 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 322 497 A1 Description [0001] The present invention relates to ionic liquids and uses thereof. The invention also provides processes for the manufacture of ionic liquids. 5 [0002] Ionic liquids are compounds which are composed of ions but which have a melting point below ambient tem- perature. -

Fcc-12-Index.Pdf
FCC 12 Index / Allyl Butanoate / I-1 Index Index Titles of monographs are shown in the boldface type. A 2-Acetyl Thiazole, 17 Alcohol C-8, 843 Acetyl Valeryl, 551 Alcohol C-9, 834 Abbreviations, 6 Acetyl Value, 1408 Alcohol C-10, 355 Absolute Alcohol (Reagent), 5 Achilleic Acid, 24 Alcohol C-11, 1202 Acacia, 546 Acid (Reagent), 5 Alcohol C-12, 664 ªAccuracyº, Defined, 1575 Acid-Hydrolyzed Milk Protein, 21 Alcohol C-16, 558 Acesulfame K, 9 Acid-Hydrolyzed Proteins, 21 Alcohol Content of Ethyl Oxyhydrate Acesulfame Potassium, 9 Acid Calcium Phosphate, 211 Flavor Chemicals (Other than Acetal, 10 Acid Hydrolysates of Proteins, 21 Essential Oils), 1455 Acetaldehyde, 10 Acidic Sodium Aluminum Phosphate, Alcohol, Diluted, 1560 Acetaldehyde Diethyl Acetal, 10 1043 Alcoholic Potassium Hydroxide TS, Acetaldehyde Test Paper, 1571 Acidified Sodium Chlorite 1560 Acetals (Essential Oils and Flavors), Solutions, 23 Alcoholometric Table, 1682 1401 Acidity Determination by Iodometric Aldehyde C-6, 560 Acetanisole, 11 Method, 1455 Aldehyde C-7, 550 Acetate C-10, 354 Acid Magnesium Phosphate, 713 Aldehyde C-8, 837 Acetate Identification Test, 1316 Acid Number (Rosins and Related Aldehyde C-9, 828 Aceteugenol, 456 Substances), 1432 Aldehyde C-10, 351 Acetic Acid Furfurylester, 494 Acid Phosphatase Activity, 1369 Aldehyde C-11 Undecyclic, 1197 Acetic Acid, Glacial, 12 Acid Phthalate Buffer, 1559 Aldehyde C-11 Undecylenic, 1200 Acetic Acid TS, Diluted, 1560 Acid Sodium Pyrophosphate, 1040 Aldehyde C-12, 665 Acetic Acid TS, Strong, 1560 Acid Trisodium Pyrophosphate, -
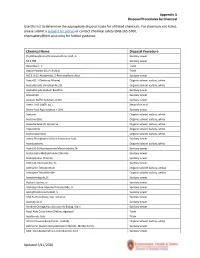
Appendix a Disposal Procedures by Chemical Updated 5/11/2020 Use
Appendix A Disposal Procedures by Chemical Use this list to determine the appropriate disposal route for all listed chemicals. For chemicals not listed, please submit a request for pickup or contact chemical safety (608-265-5700, [email protected]) for further guidance. Chemical Name Disposal Procedure (Cyclohexylamino)Propanesulfonic Acid, 3- Sanitary sewer 50 X TBE Sanitary sewer Absorbosil - 1 Trash Acacia Powder (Gum Arabic) Trash ACES, N-(2-Acetamido)-2-Aminosulfonic Acid Sanitary sewer Acetal (1,1-Diethoxy Ethane) Organic solvent carboy, white Acetaldehyde Dimethyl Acetal Organic solvent carboy, white Acetaldehyde Sodium Bisulfite Sanitary sewer Acetamide Sanitary sewer Acetate Buffer Solution, 0.2M Sanitary sewer Acetic Acid (<80% aq.) Neutralize acid Acetic Acid Aqu.Solution < 30% Sanitary sewer Acetone Organic solvent carboy, white Acetone (D6) Organic solvent carboy, white Acetone Ketal Of Glycerine Organic solvent carboy, white Acetonitrile Organic solvent carboy, white Acetonylacetone Organic solvent carboy, white Acetyl Phosphate (Lithium Potassium Salt) Sanitary sewer Acetylacetone Organic solvent carboy, white Acetyl-B-D-Mannosamine Monohydrate, N- Sanitary sewer Acetyl-beta-Methylcholine Chloride Sanitary sewer Acetylcholine Chloride Sanitary sewer Acetyl-D-Glucosamine, N- Sanitary sewer Acetylene Tetrabromide Organic solvent carboy, yellow Acetylene Tetrachloride Organic solvent carboy, yellow Acetylimidazole, N- Sanitary sewer Acetyl-L-Serine, o- Sanitary sewer Acetylpyridine Adenine Dinucleotide, 3- Sanitary sewer -

(12) United States Patent (10) Patent No.: US 9,328,220 B2 Walker (45) Date of Patent: May 3, 2016
USOO9328220B2 (12) United States Patent (10) Patent No.: US 9,328,220 B2 Walker (45) Date of Patent: May 3, 2016 (54) LIQUIDS 5,371,166 A 12/1994 Farkas et al. 5,464,880 A 11/1995 Weber et al. 6,361,940 B1 3/2002 Van Ness et al. (71) Applicant: INNOVIA FILMS LIMITED, Cumbria 6,472,565 B1 10/2002 Bahrmann et al. (GB) 6,506,239 B1 1/2003 Osumi et al. 6,900,313 B2 5/2005 Wasserscheid et al. (72) Inventor: Adam John Walker, Lincolnshire (GB) 7,167,353 B2 1/2007 Yuyama et al. 7,754.462 B2 * 7/2010 Kragl ........................ C12P1/OO (73) Assignee: Innovia Films Limited, Cumbria (GB) 252,364 8,784.686 B2 * 7/2014 Walker ................... B01J 31,003 252,364 (*) Notice: Subject to any disclaimer, the term of this 8,992,798 B2* 3/2015 Walker ................... B01J 31,003 patent is extended or adjusted under 35 252,364 U.S.C. 154(b) by 151 days. 2003. O149264 A1 8, 2003 Wasserscheid et al. 2003/0232844 A1* 12/2003 Rogier, Jr. ............ CO7D 215.54 514,260.1 (21) Appl. No.: 14/191,750 2007/0185.330 A1 8, 2007 Walker 2008. O191170 A1* 8, 2008 Walker ................... B01J 31,003 (22) Filed: Feb. 27, 2014 252,364 2008, 0221361 A1* 9, 2008 Walker ................... B01J 31,003 (65) Prior Publication Data 564,508 2008/0258113 A1 10/2008 Clyburne et al. US 2014/O187684 A1 Jul. 3, 2014 2011/O257.433 A1 10/2011 Walker Related U.S. Application Data FOREIGN PATENT DOCUMENTS (63) Continuation of application No.