Effect of Cinnamon on Glucose Control and Lipid Parameters
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bab 1 Pendahuluan
BAB 1 PENDAHULUAN Bab ini akan membahas pengertian dasar statistik dengan sub-sub pokok bahasan sebagai berikut : Sub Bab Pokok Bahasan A. Sejarah dan Perkembangan Statistik B. Tokoh-tokoh Kontributor Statistika C. Definisi dan Konsep Statistik Modern D. Kegunaan Statistik E. Pembagian Statistik F. Statistik dan Komputer G. Soal Latihan A. Sejarah dan Perkembangan Statistik Penggunaan istilah statistika berakar dari istilah-istilah dalam bahasa latin modern statisticum collegium (“dewan negara”) dan bahasa Italia statista (“negarawan” atau “politikus”). Istilah statistik pertama kali digunakan oleh Gottfried Achenwall (1719-1772), seorang guru besar dari Universitas Marlborough dan Gottingen. Gottfried Achenwall (1749) menggunakan Statistik dalam bahasa Jerman untuk pertama kalinya sebagai nama bagi kegiatan analisis data kenegaraan, dengan mengartikannya sebagai “ilmu tentang negara/state”. Pada awal abad ke- 19 telah terjadi pergeseran arti menjadi “ilmu mengenai pengumpulan dan klasifikasi data”. Sir John Sinclair memperkenalkan nama dan pengertian statistics ini ke dalam bahasa Inggris. E.A.W. Zimmerman mengenalkan kata statistics ke negeri Inggris. Kata statistics dipopulerkan di Inggris oleh Sir John Sinclair dalam karyanya: Statistical Account of Scotland 1791-1799. Namun demikian, jauh sebelum abad XVIII masyarakat telah mencatat dan menggunakan data untuk keperluan mereka. Pada awalnya statistika hanya mengurus data yang dipakai lembaga- lembaga administratif dan pemerintahan. Pengumpulan data terus berlanjut, khususnya melalui sensus yang dilakukan secara teratur untuk memberi informasi kependudukan yang selalu berubah. Dalam bidang pemerintahan, statistik telah digunakan seiring dengan perjalanan sejarah sejak jaman dahulu. Kitab perjanjian lama (old testament) mencatat adanya kegiatan sensus penduduk. Pemerintah kuno Babilonia, Mesir, dan Roma mengumpulkan data lengkap tentang penduduk dan kekayaan alam yang dimilikinya. -

Stock Market Prediction Using Ensemble of Graph Theory, Machine Learning and Deep Learning Models
San Jose State University SJSU ScholarWorks Master's Projects Master's Theses and Graduate Research Spring 5-20-2019 STOCK MARKET PREDICTION USING ENSEMBLE OF GRAPH THEORY, MACHINE LEARNING AND DEEP LEARNING MODELS Pratik Patil San Jose State University Follow this and additional works at: https://scholarworks.sjsu.edu/etd_projects Part of the Artificial Intelligence and Robotics Commons, and the Other Computer Sciences Commons Recommended Citation Patil, Pratik, "STOCK MARKET PREDICTION USING ENSEMBLE OF GRAPH THEORY, MACHINE LEARNING AND DEEP LEARNING MODELS" (2019). Master's Projects. 692. DOI: https://doi.org/10.31979/etd.38nc-j52r https://scholarworks.sjsu.edu/etd_projects/692 This Master's Project is brought to you for free and open access by the Master's Theses and Graduate Research at SJSU ScholarWorks. It has been accepted for inclusion in Master's Projects by an authorized administrator of SJSU ScholarWorks. For more information, please contact [email protected]. STOCK MARKET PREDICTION USING ENSEMBLE OF GRAPH THEORY, MACHINE LEARNING AND DEEP LEARNING MODELS A Project Report Presented to Dr. Ching seh Wu Department of Computer Science San José State University In Partial Fulfillment Of the Requirements for the Class CS 298 By Pratik Patil May 2019 © 2019 Pratik Patil ALL RIGHTS RESERVED The Designated Thesis Committee Approves the Thesis Titled STOCK MARKET PREDICTION USING ENSEMBLE OF GRAPH THEORY, MACHINE LEARNING AND DEEP LEARNING MODELS by Pratik Patil APPROVED FOR THE DEPARTMENT OF COMPUTER SCIENCE SAN JOSÉ STATE UNIVERSITY May 2019 Dr. Ching seh Wu Department of Computer Science Dr. Katerina Potika Department of Computer Science Dr. Marjan Orang Department of Economics ACKNOWLEDGEMENT This has been one long and arduous journey, but nevertheless a worthwhile life experience because of the many great Professors at SJSU and beloved friends. -

Insight MFR By
Manufacturers, Publishers and Suppliers by Product Category 11/6/2017 10/100 Hubs & Switches ASCEND COMMUNICATIONS CIS SECURE COMPUTING INC DIGIUM GEAR HEAD 1 TRIPPLITE ASUS Cisco Press D‐LINK SYSTEMS GEFEN 1VISION SOFTWARE ATEN TECHNOLOGY CISCO SYSTEMS DUALCOMM TECHNOLOGY, INC. GEIST 3COM ATLAS SOUND CLEAR CUBE DYCONN GEOVISION INC. 4XEM CORP. ATLONA CLEARSOUNDS DYNEX PRODUCTS GIGAFAST 8E6 TECHNOLOGIES ATTO TECHNOLOGY CNET TECHNOLOGY EATON GIGAMON SYSTEMS LLC AAXEON TECHNOLOGIES LLC. AUDIOCODES, INC. CODE GREEN NETWORKS E‐CORPORATEGIFTS.COM, INC. GLOBAL MARKETING ACCELL AUDIOVOX CODI INC EDGECORE GOLDENRAM ACCELLION AVAYA COMMAND COMMUNICATIONS EDITSHARE LLC GREAT BAY SOFTWARE INC. ACER AMERICA AVENVIEW CORP COMMUNICATION DEVICES INC. EMC GRIFFIN TECHNOLOGY ACTI CORPORATION AVOCENT COMNET ENDACE USA H3C Technology ADAPTEC AVOCENT‐EMERSON COMPELLENT ENGENIUS HALL RESEARCH ADC KENTROX AVTECH CORPORATION COMPREHENSIVE CABLE ENTERASYS NETWORKS HAVIS SHIELD ADC TELECOMMUNICATIONS AXIOM MEMORY COMPU‐CALL, INC EPIPHAN SYSTEMS HAWKING TECHNOLOGY ADDERTECHNOLOGY AXIS COMMUNICATIONS COMPUTER LAB EQUINOX SYSTEMS HERITAGE TRAVELWARE ADD‐ON COMPUTER PERIPHERALS AZIO CORPORATION COMPUTERLINKS ETHERNET DIRECT HEWLETT PACKARD ENTERPRISE ADDON STORE B & B ELECTRONICS COMTROL ETHERWAN HIKVISION DIGITAL TECHNOLOGY CO. LT ADESSO BELDEN CONNECTGEAR EVANS CONSOLES HITACHI ADTRAN BELKIN COMPONENTS CONNECTPRO EVGA.COM HITACHI DATA SYSTEMS ADVANTECH AUTOMATION CORP. BIDUL & CO CONSTANT TECHNOLOGIES INC Exablaze HOO TOO INC AEROHIVE NETWORKS BLACK BOX COOL GEAR EXACQ TECHNOLOGIES INC HP AJA VIDEO SYSTEMS BLACKMAGIC DESIGN USA CP TECHNOLOGIES EXFO INC HP INC ALCATEL BLADE NETWORK TECHNOLOGIES CPS EXTREME NETWORKS HUAWEI ALCATEL LUCENT BLONDER TONGUE LABORATORIES CREATIVE LABS EXTRON HUAWEI SYMANTEC TECHNOLOGIES ALLIED TELESIS BLUE COAT SYSTEMS CRESTRON ELECTRONICS F5 NETWORKS IBM ALLOY COMPUTER PRODUCTS LLC BOSCH SECURITY CTC UNION TECHNOLOGIES CO FELLOWES ICOMTECH INC ALTINEX, INC. -

Statistický Software
Statistický software 1 Software AcaStat GAUSS MRDCL RATS StatsDirect ADaMSoft GAUSS NCSS RKWard[4] Statistix Analyse-it GenStat OpenEpi SalStat SYSTAT The ASReml GoldenHelix Origin SAS Unscrambler Oxprogramming Auguri gretl language SOCR UNISTAT BioStat JMP OxMetrics Stata VisualStat BrightStat MacAnova Origin Statgraphics Winpepi Dataplot Mathematica Partek STATISTICA WinSPC EasyReg Matlab Primer StatIt XLStat EpiInfo MedCalc PSPP StatPlus XploRe EViews modelQED R SPlus Excel Minitab R Commander[4] SPSS 2 Data miningovýsoftware n Cca 20 až30 dodavatelů n Hlavníhráči na trhu: n Clementine, IBM SPSS Modeler n IBM’s Intelligent Miner, (PASW Modeler) n SGI’sMineSet, n SAS’s Enterprise Miner. n Řada vestavěných produktů: n fraud detection: n electronic commerce applications, n health care, n customer relationship management 3 Software-SAS n : www.sas.com 4 SAS n Společnost SAS Institute n Vznik 1976 v univerzitním prostředí n Dnes:největšísoukromásoftwarováspolečnost na světě (více než11.000 zaměstnanců) n přes 45.000 instalací n cca 9 milionů uživatelů ve 118 zemích n v USA okolo 1.000 akademických zákazníků (SAS používávětšina vyšších a vysokých škol a výzkumných pracovišť) 5 SAS 6 SAS 7 SAS q Statistickáanalýza: Ø Popisnástatistika Ø Analýza kontingenčních (frekvenčních) tabulek Ø Regresní, korelační, kovariančníanalýza Ø Logistickáregrese Ø Analýza rozptylu Ø Testováníhypotéz Ø Diskriminačníanalýza Ø Shlukováanalýza Ø Analýza přežití Ø … 8 SAS q Analýza časových řad: Ø Regresnímodely Ø Modely se sezónními faktory Ø Autoregresnímodely Ø -

The R Project for Statistical Computing a Free Software Environment For
The R Project for Statistical Computing A free software environment for statistical computing and graphics that runs on a wide variety of UNIX platforms, Windows and MacOS OpenStat OpenStat is a general-purpose statistics package that you can download and install for free. It was originally written as an aid in the teaching of statistics to students enrolled in a social science program. It has been expanded to provide procedures useful in a wide variety of disciplines. It has a similar interface to SPSS SOFA A basic, user-friendly, open-source statistics, analysis, and reporting package PSPP PSPP is a program for statistical analysis of sampled data. It is a free replacement for the proprietary program SPSS, and appears very similar to it with a few exceptions TANAGRA A free, open-source, easy to use data-mining package PAST PAST is a package created with the palaeontologist in mind but has been adopted by users in other disciplines. It’s easy to use and includes a large selection of common statistical, plotting and modelling functions AnSWR AnSWR is a software system for coordinating and conducting large-scale, team-based analysis projects that integrate qualitative and quantitative techniques MIX An Excel-based tool for meta-analysis Free Statistical Software This page links to free software packages that you can download and install on your computer from StatPages.org Free Statistical Software This page links to free software packages that you can download and install on your computer from freestatistics.info Free Software Information and links from the Resources for Methods in Evaluation and Social Research site You can sort the table below by clicking on the column names. -
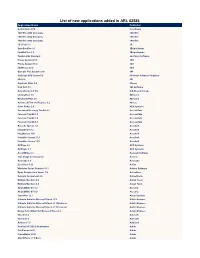
List of New Applications Added in ARL #2586
List of new applications added in ARL #2586 Application Name Publisher NetCmdlets 2016 /n software 1099 Pro 2009 Corporate 1099 Pro 1099 Pro 2020 Enterprise 1099 Pro 1099 Pro 2008 Corporate 1099 Pro 1E Client 5.1 1E SyncBackPro 9.1 2BrightSparks FindOnClick 2.5 2BrightSparks TaxAct 2002 Standard 2nd Story Software Phone System 15.5 3CX Phone System 16.0 3CX 3CXPhone 16.3 3CX Grouper Plus System 2021 3M CoDeSys OPC Server 3.1 3S-Smart Software Solutions 4D 15.0 4D Duplicate Killer 3.4 4Team Disk Drill 4.1 508 Software NotesHolder 2.3 Pro A!K Research Labs LibraryView 1.0 AB Sciex MetabolitePilot 2.0 AB Sciex Advanced Find and Replace 5.2 Abacre Color Picker 2.0 ACA Systems Password Recovery Toolkit 8.2 AccessData Forensic Toolkit 6.0 AccessData Forensic Toolkit 7.0 AccessData Forensic Toolkit 6.3 AccessData Barcode Xpress 7.0 AccuSoft ImageGear 17.2 AccuSoft ImagXpress 13.6 AccuSoft PrizmDoc Server 13.1 AccuSoft PrizmDoc Server 12.3 AccuSoft ACDSee 2.2 ACD Systems ACDSync 1.1 ACD Systems Ace Utilities 6.3 Acelogix Software True Image for Crucial 23. Acronis Acrosync 1.6 Acrosync Zen Client 5.10 Actian Windows Forms Controls 16.1 Actipro Software Opus Composition Server 7.0 ActiveDocs Network Component 4.6 ActiveXperts Multiple Monitors 8.3 Actual Tools Multiple Monitors 8.8 Actual Tools ACUCOBOL-GT 5.2 Acucorp ACUCOBOL-GT 8.0 Acucorp TransMac 12.1 Acute Systems Ultimate Suite for Microsoft Excel 13.2 Add-in Express Ultimate Suite for Microsoft Excel 21.1 Business Add-in Express Ultimate Suite for Microsoft Excel 21.1 Personal Add-in Express -

Cumulation of Poverty Measures: the Theory Beyond It, Possible Applications and Software Developed
Cumulation of Poverty measures: the theory beyond it, possible applications and software developed (Francesca Gagliardi and Giulio Tarditi) Siena, October 6th , 2010 1 Context and scope Reliable indicators of poverty and social exclusion are an essential monitoring tool. In the EU-wide context, these indicators are most useful when they are comparable across countries and over time. Furthermore, policy research and application require statistics disaggregated to increasingly lower levels and smaller subpopulations. Direct, one-time estimates from surveys designed primarily to meet national needs tend to be insufficiently precise for meeting these new policy needs. This is particularly true in the domain of poverty and social exclusion, the monitoring of which requires complex distributional statistics – statistics necessarily based on intensive and relatively small- scale surveys of households and persons. This work addresses some statistical aspects relating to improving the sampling precision of such indicators in EU countries, in particular through the cumulation of data over rounds of regularly repeated national surveys. 2 EU-SILC The reference data for this purpose are EU Statistics on Income and Living Conditions, the major source of comparative statistics on income and living conditions in Europe. A standard integrated design has been adopted by nearly all EU countries. It involves a rotational panel, with a new sample of households and persons introduced each year to replace one-fourth of the existing sample. Persons enumerated in each new sample are followed-up in the survey for four years. The design yields each year a cross- sectional sample, as well as longitudinal samples of 2, 3 and 4 year duration. -

Comparison of Three Common Statistical Programs Available to Washington State County Assessors: SAS, SPSS and NCSS
Washington State Department of Revenue Comparison of Three Common Statistical Programs Available to Washington State County Assessors: SAS, SPSS and NCSS February 2008 Abstract: This summary compares three common statistical software packages available to county assessors in Washington State. This includes SAS, SPSS and NCSS. The majority of the summary is formatted in tables which allow the reader to more easily make comparisons. Information was collected from various sources and in some cases includes opinions on software performance, features and their strengths and weaknesses. This summary was written for Department of Revenue employees and county assessors to compare statistical software packages used by county assessors and as a result should not be used as a general comparison of the software packages. Information not included in this summary includes the support infrastructure, some detailed features (that are not described in this summary) and types of users. Contents General software information.............................................................................................. 3 Statistics and Procedures Components................................................................................ 8 Cost Estimates ................................................................................................................... 10 Other Statistics Software ................................................................................................... 13 General software information Information in this section was -

Statistika Penelitian Bisnis & Manajemen
Statistika Penelitian Bisnis & Manajemen Statistik Parametrik, Non-Parametrik, Regresi Linier, Analisis Jalur dan SEM Oleh : Prof. Dr. H. Siswoyo Haryono, MM, MPd. Penyusun : Dwi Puryanto, SE, MM ISBN : Cetakan pertama: 2020 Penerbit LP3M UMY Universitas Muhammadiyah Yogyakarta Hak Cipta dilindungi Undang-undang. Dilarang mengutip atau memperbanyak sebagian atau seluruh buku dalam bentuk apapun, tanpa ijin tertulis dari penerbit. Statistika Penelitian Manajemen i Prakata Pertama-tama penulis panjatkan puji dan syukur kehadirat Tuhan YME, Allah SWT, bahwa pada akhirnya buku ini dapat diterbitkan. Tujuan diterbitkannya buku ini adalah untuk membantu para mahasiswa dalam mempelajari Statistika (Ilmu Statistik) khususnya dalam aplikasi penelitian bidang manajemen. Mengingat statistik pada saat ini telah banyak memanfaatkan program-program computer, diantaranya SPSS, dan SEM maka buku ini diberi judul Statistika Penelitian Bisnis dan Manajemen (Statistik Parametrik, Non-Parametrik, Regresi Linier, Analisis Jalur dan SEM). Sasaran pembaca buku ini adalah mahasiswa Program Sarjana Strata Satu (S-1) Magister (S-2) dan Doktor (S-3) yang sedang menyiapkan diri menulis Skripsi, Tesis atau Disertasi. Sasaran lain adalah para manajer atau praktisi bisnis yang berminat memperdalam ilmu Penelitian di bidang Bisnis dan Manajemen. Pada kesempatan ini penulis menyampaikan ucapan terimakasih dan penghargaan yang tulus kepada semua pihak yang telah membantu tersusunnya buku ini. Penulis menyadari bahwa masih terdapat kekurangsempurnaan dalam penulisan buku -
The Development of a Statistical Software Resource for Medical Research
The University of Manchester Research The development of a statistical software resource for medical research Link to publication record in Manchester Research Explorer Citation for published version (APA): Buchan, I. E. (2000). The development of a statistical software resource for medical research. University of Liverpool. Citing this paper Please note that where the full-text provided on Manchester Research Explorer is the Author Accepted Manuscript or Proof version this may differ from the final Published version. If citing, it is advised that you check and use the publisher's definitive version. General rights Copyright and moral rights for the publications made accessible in the Research Explorer are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. Takedown policy If you believe that this document breaches copyright please refer to the University of Manchester’s Takedown Procedures [http://man.ac.uk/04Y6Bo] or contact [email protected] providing relevant details, so we can investigate your claim. Download date:04. Oct. 2021 The Development of a Statistical Computer Software Resource for Medical Research Thesis submitted in accordance with the requirements of the University of Liverpool For the degree of Doctor of Medicine By Iain Edward Buchan November 2000 Liverpool, England To my parents… PREFACE Preface Declaration This thesis is the result of my own work. The material contained in this thesis has not been presented, nor is currently being presented, either wholly or in part for any other degree or other qualification. -

Medical Statistics Online Help: Sample Size & Power for Clinical Trials
Medical statistics online help: Sample size & power for clinical trials Clinical Trials: an Sample size Dedicated software Reference texts overview calculation It is very important that the design of a clinical trial has been thoroughly considered before it is undertaken. A crucial component of the design is the number of participants required, in order to reliably answer the clinical question. The aim of these pages is to clarify some of the key issues regarding sample size and power. Clinical Trials: an overview A Clinical Trial is a carefully planned experiment involving human participants, which attempts to answer a pre-defined set of research questions with respect to an intervention. Types of clinical trials include: · Intervention or therapeutic - e.g. a drug treatment for stroke; a surgical intervention such as simple mastectomy for breast cancer · Preventative - e.g. screening for cervical cancer; educational methods and lifestyle interventions; vaccine trials for TB top Sample size When planning a clinical trial, it is very important to consider how many participants you will need to reliably answer the clinical question. Too many participants is a needless waste of resources (and possibly lives), which could result in a beneficial treatment being denied to patients unnecessarily. Too few participants will not produce a precise, reliable and definitive answer, which can also be considered unethical. Under this common scenario, patients might be denied a useful treatment because the trials were frequently underpowered (i.e. too small to detect a treatment effect) – this can also result in further studies being cancelled without good reason. Choosing a sample size is a combination of logistical and pragmatic considerations. -

Syllabus Personal Introduction
SYLLABUS Lamar University, a Member of The Texas State University System, is accredited by the Commission on Colleges of the Southern Association of Colleges and Schools to award Associate, Baccalaureate, Masters, and Doctorate degrees (for more information go to http://www.lamar.edu). Course Title: Biostatistics Course Number: HLTH 5303 Course Section: 48F Department: Health and Kinesiology Professor: Dr. Israel Msengi, CHES Office Hours: Virtual (online) Wednesday 7-8pm or by appointment. Physical: MW 11:30-2:00pm Contact Information: LU email: [email protected] Office:HHP 211 Phone: 409-880-8716 PERSONAL INTRODUCTION Welcome to Lamar University. My name is Dr. Israel Msengi, and I will be your instructor of record for Biostatistics. By way of a very brief introduction, I earned my baccalaureate in Public Administration with emphasis on Governance and master’s degrees in Community Health and a Doctorate in Community and Public Health. My area of expertise is Environmental Health, but I also enjoy the challenges of Obesogenic Environment research. I joined the faculty at Lamar in the fall of 2008 and I am currently an associate professor for the Department of Health and Kinesiology in the College of Education and Human Development. COURSE DESCRIPTION Biostatistics is an area of statistics that covers and provides the specialized methodology for collecting and analyzing biomedical, health care, and public health data. This course meets the biostatistics core course requirement for all degrees and concentrations in the Public Health program. Presentation of the principles and methods of data description and elementary parametric and non-parametric statistical analysis as well as sample size estimation are covered.